Abstract
Inbreeding can have negative effects on survival and reproduction, which may be of conservation concern in small and isolated populations. However, the physiological mechanisms underlying inbreeding depression are not well-known. The length of telomeres, the DNA sequences protecting chromosome ends, has been associated with health or fitness in several species. We investigated effects of inbreeding on early-life telomere length in two small island populations of wild house sparrows (Passer domesticus) known to be affected by inbreeding depression. Using genomic measures of inbreeding we found that inbred nestling house sparrows (n = 371) have significantly shorter telomeres. Using pedigree-based estimates of inbreeding we found a tendency for inbred nestling house sparrows to have shorter telomeres (n = 1195). This negative effect of inbreeding on telomere length may have been complemented by a heterosis effect resulting in longer telomeres in individuals that were less inbred than the population average. Furthermore, we found some evidence of stronger effects of inbreeding on telomere length in males than females. Thus, telomere length may reveal subtle costs of inbreeding in the wild and demonstrate a route by which inbreeding negatively impacts the physiological state of an organism already at early life-history stages.
Similar content being viewed by others
Introduction
Inbreeding has significant detrimental effects on survival, reproduction, and resistance to disease and other stressors in wild populations (Keller and Waller 2002). Such decline in fitness resulting from an increase in genome-wide homozygosity is known as inbreeding depression (Charlesworth and Willis 2009) and is of major concern in small and isolated populations, in particular of endangered species (Bozzuto et al. 2019; Harrisson et al. 2019; Hedrick and Kalinowski 2000). Increased homozygosity can lead to reduced fitness due to expression of deleterious recessive alleles (“dominance hypothesis”) or increased homozygosity at loci with heterozygote advantage (“overdominance hypothesis”, Charlesworth and Willis 2009). Regardless of the genetic basis for inbreeding depression, it is difficult to identify and quantify the physiological mechanisms underlying the fitness costs of inbreeding (Fox and Reed 2011; Kristensen et al. 2010; Losdat et al. 2016).
Telomeres are short DNA tandem repeats that are found at the tips of most eukaryotic chromosomes (Blackburn and Gall 1978; Červenák et al. 2021). Telomeres shorten during cell division (Harley et al. 1990), but may also shorten due to several other reasons including physiological processes generating oxidative stress (Barnes et al. 2019; Monaghan and Ozanne 2018; Reichert and Stier 2017; von Zglinicki 2002). The high guanine content of telomeres (50%) makes them particularly vulnerable to oxidative stress (Kawanishi and Oikawa 2004). Short telomeres can trigger apoptosis and telomere attrition is considered a hallmark of aging (López-Otín et al. 2013), although the causal involvement of telomere shortening in organismal senescence is not well understood (Simons 2015). However, telomere length (TL) may reflect the cumulative stress experienced by an individual (Bateson 2016; Monaghan 2014), and TL or TL shortening are associated with health or fitness in several species (Barrett et al. 2013; Chatelain et al. 2020; Froy et al. 2021; Heidinger et al. 2021; Wilbourn et al. 2018). Thus, TL is increasingly used as a biomarker of somatic integrity in studies of physiological or evolutionary ecology (Bateson and Poirier 2019; Haussmann and Marchetto 2010; Pepper et al. 2018; Young 2018).
Inbreeding depression can be caused by reduced immune response (Charpentier et al. 2008; Reid et al. 2003) and higher maintenance metabolism (Ketola and Kotiaho 2009), which increases oxidative stress de Boer et al. 2018a; Okada et al. 2011). Thus, inbred individuals may experience higher levels of oxidative stress (Kristensen et al. 2005; Pedersen et al. 2008) and thus have shorter telomeres (von Zglinicki 2002). We therefore hypothesize that TL could provide an integrative measure of the somatic costs associated with inbreeding depression in wild populations, with inbred individuals having shorter telomeres than outbred individuals. However, the few studies investigating associations between inbreeding and TL have found equivocal results. In line with our expectations, Bebbington et al. (2016) found that homozygosity was negatively associated with TL in wild Seychelles warblers (Acrocephalus sechellensis) and Seluanov et al. (2008) reported that telomeres were shorter in inbred laboratory strains of Norway rats (Rattus norvegicus) in captivity compared to a single wild-caught rat. Many domesticated species are generally assumed to be more inbred than their wild counterparts (Bosse et al. 2018; Moyers et al. 2018; Wiener and Wilkinson 2011). However, several studies have found that telomeres were longer in inbred domesticated strains of laboratory mice (Mus spp. and Peromyscus spp., Hemann and Greider 2000; Manning et al. 2002; Seluanov et al. 2008), in domesticated strains of pearl millet (Pennisetum glaucum, Sridevi et al. 2002), in domesticated inbred chicken (Gallus gallus, O’Hare and Delany 2009), and across several species of domesticated mammals (Pepke and Eisenberg 2021) compared to non-domesticated species. However, there were no clear differences in TL between inbred and wild leporid strains (Forsyth et al. 2005). Other studies found no association between pedigree-based inbreeding coefficients and TL or telomere attrition in humans (Homo sapiens, Mansour et al. 2011), wild sand lizards (Lacerta agilis, Olsson et al. 2018), or wild natterjack toads (Epidalea calamita, Sánchez-Montes et al. 2020). Becker et al. (2015) reported a weak non-significant but positive association between inbreeding and TL in wild white-throated dippers (Cinclus cinclus).
These contrasting results suggest that the telomere dynamics of captive, domesticated species living in a controlled environment may not be representative of wild, free-living populations (Chatelain et al. 2020; Pepke and Eisenberg 2021; Weinstein and Ciszek 2002). For instance, captive populations may be less vulnerable to inbreeding because inbreeding depression is greater under stressful environmental conditions (Fox and Reed 2011; Reed et al. 2002). Furthermore, captivity may in itself provide conditions that change the telomere dynamics of the populations (Eisenberg 2011), e.g. Hemann and Greider (2000) attributed the longer telomeres of inbred mice to effects of captive breeding and not inbreeding per se. For instance, TL shortening rates may increase during metabolically costly processes such as reproduction (Sudyka et al. 2019; Wood et al. 2021) and inbreeding may reduce fecundity (Keller and Waller 2002). Such effects have been suggested to explain the observation of longer adult TL in some inbred domesticated species (Eisenberg 2011), which could be resolved by measuring TL in early-life. Furthermore, most of the studies of domesticated animals compared TLs of different populations or species and their results may not be extrapolated to natural variation in TL and inbreeding levels within wild populations. Indeed, TL can vary considerably within species (Tricola et al. 2018) and across closely related species (Pepke et al. 2021c) in the wild. Finally, it is not known if outbreeding could be accompanied by a heterosis effect (hybrid vigor, e.g. Charlesworth and Willis 2009) acting on TL. Physiological mechanisms underlying heterosis are not well-known (Wu et al. 2021), but we hypothesize that the observed fitness benefits of outcrossing inbred populations (Frankham 2015) could be reflected in TL restoration (Nuzhdin and Reiwitch 2002; Ozawa et al. 2019).
In this study, we utilized a long-term metapopulation study to examine how inbreeding affects early-life TL in wild house sparrows (Passer domesticus). Inbreeding has been shown to reduce fitness components such as recruitment probability, adult lifespan, and both annual and lifetime reproductive success in this metapopulation (Billing et al. 2012; Jensen et al. 2007; Niskanen et al. 2020), but the physiological effects underlying these phenomena remain unknown. We expect that inbred individuals will have shorter telomeres if TL is a general biomarker of somatic integrity and health (e.g. Bebbington et al. 2016; Boonekamp et al. 2013; Wilbourn et al. 2018). The effects of inbreeding on TL might be sex-specific Benton et al. 2018; Billing et al. 2012; de Boer et al. 2018a; de Boer, Eens, & MülleBoer et al. 2018b) or depend on environmental conditions (Armbruster and Reed 2005; Szulkin and Sheldon 2007). However, TL is negatively associated with body size or growth rate within many species (Monaghan and Ozanne 2018; Ringsby et al. 2015) and may change with age (Hall et al. 2004; Remot et al. 2021) or vary between sexes (Barrett and Richardson 2011; Remot et al. 2020) and habitat quality (Angelier et al. 2013; McLennan et al. 2021; Wilbourn et al. 2017). We therefore account for body size (measured as tarsus length), age, sex, and habitat type, and test for an interaction between inbreeding levels and sex or habitat type, when investigating the association between TL and inbreeding. We use three different measures of inbreeding; marker-based estimates (n = 371) which are better at capturing homozygosity and inbreeding caused by distant ancestors not included in a pedigree, and pedigree-based estimates (Kardos et al. 2016) for which larger samples size may be obtained from long-term field studies (n = 1195). Finally, to investigate a potential heterosis effect on TL, we test if the association between TL and inbreeding is different among outbred and inbred individuals.
Materials and methods
Study system
This study was conducted in two natural populations of house sparrows in northern Norway. On the island of Hestmannøy (66°33’N, 12°50‘E), the sparrows live around dairy farms, where they nest inside barns in cavities or nest boxes. The island is characterized by cultivated grassland, mountains, forest, and heathland. On the island of Træna (66°30’N, 12°05‘E), 34 km further from the mainland, the sparrows live in gardens of a small human settlement and nest in nest boxes. This island is dominated by heathland, sparse forest, and gardens. The natural breeding environment for house sparrows is human habitation (Hanson et al. 2020) and they have evolved their commensal relationship with humans for millennia (Ravinet et al. 2018). While human presence or farming provide the natural basis of existence for house sparrows (Ringsby et al. 2006), demographic characteristics, breeding densities, and inbreeding rates are comparable to other small isolated wild animal populations (Araya-Ajoy et al. 2021; Jensen et al. 2007; Niskanen et al. 2020). In the years 1994–2013 (on Hestmannøy) and 2004–2013 (on Træna), nestlings at the age of 5–14 days were ringed with a unique combination of color rings for identification. Nestlings were also blood sampled by brachial venipuncture, and tarsometatarsus (tarsus) was measured with slide calipers to the nearest 0.01 mm. Tarsus length is here used as an index of body size (Rising and Somers 1989; Senar and Pascual 1997). Blood samples (25 µL) were stored in 96% ethanol at room temperature in the field and at -20 °C in the laboratory until DNA extraction (described in Pepke et al. 2021b). Birds that were resighted or recaptured in the year following hatching (i.e. from 1995 to 2014 on Hestmannøy and from 2005 to 2014 on Træna) were categorized as first-year survivors (i.e. recruits).
Telomere length measurements
Relative erythrocyte telomere length (TL) was successfully measured in DNA derived from 2746 whole blood samples from house sparrow nestlings using the qPCR method (Cawthon 2002) as described in Pepke et al. (2021a). For this study, we included only individuals with two known parents and at least two known grandparents, or for which genomic inbreeding coefficients could be estimated (described below), resulting in a sample size of n = 1370 individuals (n = 679 males and n = 691 females of which n = 1161 were from Hestmannøy and n = 209 from Træna, see sample size details in Table 1). TL was determined relative to the amount of a non-variable gene (GAPDH, Criscuolo et al. 2009) and a reference sample, which was included as a two-fold serial dilution (40–2.5 ng/well) on all plates to produce a standard curve. All samples were randomized and run in triplicates on 2 × 125 96-well plates, which all included a nontarget control sample. All samples were processed within a few months by the same researcher (MLP) to reduce technical effects. Relative TL was computed using qBASE (Hellemans et al. 2007) while controlling for inter-run variation. All individual plate efficiencies were within 100 ± 10% (see Pepke et al. 2021a).
Sex was successfully determined for n = 1360 individuals by amplification of the CHD gene on the avian sex chromosomes as described in Jensen et al. (2007). For n = 10 individuals, sex was determined based on their adult plumage.
Microsatellite pedigree construction
Microsatellite (MS) pedigrees were constructed based on 13 polymorphic microsatellite markers using CERVUS 3.0 (Kalinowski et al. 2007) as described in previous studies (Billing et al. 2012; Jensen et al. 2003, 2008). The assignment of parentage was correct in at least 90% of cases (see Jensen et al. 2008). Nestlings within the same clutch were assumed to have the same mother. This metapopulation pedigree (Jensen et al. 2008) was pruned to contain n = 2184 informative ancestors (n = 1710 maternities and n = 1734 paternities), including non-phenotyped ancestors, using the R package MCMCglmm (Hadfield 2010). Maximum pedigree depth was 13 generations, the number of equivalent complete generations (i.e. the sum of the proportion of known ancestors across all generations, Wellmann 2021) was 1.834, and the mean pairwise relatedness was 0.006. We calculated inbreeding coefficients (F PED), which estimate the expected proportion of an individual’s genome that is identical by descent (IBD), based on the MS pedigree for individuals with two known parents and at least two known grandparents (n = 1057 from Hestmannøy and n = 138 from Træna, Table 1) using the R package pedigree (Coster 2012). We also selected a subset of individuals with at least two full ancestral generations (i.e. four known grandparents) to only include the most robust estimates of F PED (n = 313 from Hestmannøy and n = 7 from Træna).
Genomic inbreeding estimation
Starting from year 1997 (Hestmannøy) or 2004 (Træna), birds that survived until recruitment (n = 275 from Hestmannøy and n = 96 from Træna) were genotyped for 200,000 Single Nucleotide Polymorphisms (SNPs) as described in Lundregan et al. (2018). Two genomic inbreeding coefficients were then estimated using 118,810 autosomal SNPs not in strong linkage disequilibrium, as described in Niskanen et al. (2020).
The weighted average homozygosity over all loci from the genomic relationship matrix (F GRM) was estimated for the whole metapopulation (consisting of eight island populations) simultaneously using the GCTA software (\({\widehat{F}}^{III}\) in Yang et al. 2011). F GRM gives more weight to homozygotes of the minor allele than of the major allele, and it is an estimate of the correlation between homologous genes of the two gametes of an individual relative to the current population (Yang et al. 2011). F GRM can be negative if the probability that the two homologous genes of an individual are IBD is smaller than that of two homologous genes being drawn at random from the reference population (Wang 2014; Yang et al. 2011). Thus, the individuals with the smallest estimates of F GRM are expected to be outbred (hybrids) because of e.g. mating involving immigrants (Wang 2014). Thus, we suggest that if an association between F GRM and TL is stronger among outbred individuals (with F GRM values smaller than average) than among inbred individuals (with F GRM values larger than average), it may be partly attributed to a heterosis effect acting on TL. Alternatively, an association between F GRM and TL may be mainly driven by highly inbred individuals, or the effect of inbreeding on TL may be linear across different levels of homozygosity.
The proportion of the genome within runs-of-homozygosity (F ROH ranging from 0 to 1, McQuillan et al. 2008) was estimated using the PLINK software (Purcell et al. 2007). Homozygous sequences of minimum length of 2 Mbp were extracted using the PLINK settings: --homozyg group --homozyg-density 10 --homozyg-gap 1000 --homozyg-kb 2000 --homozyg-snp 50 --homozyg-window-het 0 --homozyg-window-missing 5 --homozyg-window-snp 50 (see Niskanen et al. 2020). ROH arise through mating of individuals that are IBD, and may therefore be used to estimate inbreeding (Curik et al. 2014). Based on the house sparrow reference genome (Elgvin et al. 2017) and linkage map (Hagen et al. 2020), homozygous sequences of 2 Mbp would be caused by inbreeding that occurred up to 12 generations ago (Niskanen et al. 2020).
Statistical analyses
To test whether TL was affected by inbreeding, we fitted linear mixed models (LMMs) using the package lme4 (Bates et al. 2015) in R v. 3.6.3 (R Core Team 2020). TL (response variable) was log10-transformed to conform to the assumption of normally distributed residuals and the models were fitted with a (continuous) fixed effect of one of the inbreeding coefficients (F PED [n = 1195], F PED with at least two full generations known [n = 320], F GRM [n = 371], or F ROH [n = 371], see Table 1 for sample size details). Since genomic estimators of inbreeding (F GRM and F ROH) were only available for recruits (first-year survivors), we tested whether the relationship between TL and F PED varied between survivors (“1”, n = 206) and non-survivors (“0”, n = 989) by including an interaction effect between F PED and first-year survival. Tarsus length increases with nestling age, so tarsus length was age-corrected by taking the residuals from a regression of tarsus length on age and age squared. This allowed us to include both tarsus length and age in the models describing variation in TL. Thus, age-standardized tarsus length, fledgling age at sampling (in number of days), hatch day (ordinal date mean centered across years), population identity (categorical: Hestmannøy or Træna), and sex (categorical: male or female) were included as fixed effects in all models. We tested whether the effect of inbreeding on TL varied between sexes and populations by including two-way interaction terms between the inbreeding coefficient and sex or population identity. Random intercepts were fitted for year and brood identity to account for the non-independence of nestlings from the same year and brood. This also controls for within-brood effects of inbreeding levels (Olsson et al. 2018). We then tested whether the inclusion of the inbreeding coefficient and interaction terms improved the baseline model (without the inbreeding coefficient) by comparing the resulting 5 candidate models using Akaike’s information criterion corrected for small sample sizes (AICc, Akaike 1973; Hurvich and Tsai 1989). Akaike weights (w) and evidence ratios (ER) were calculated to determine the relative fit of models to the data (Burnham and Anderson 2002). Models were validated visually using diagnostic plots of residuals, and model parameters are from models refitted with restricted maximum likelihood (REML). Estimates are reported with standard errors (SE) and 95% confidence intervals (CI). Regression lines were visualized using ggplot2 (Wickham 2016).
To investigate heterosis effects on TL, we tested if the slopes of the regression between F GRM and TL differed between individuals that were more inbred than on average (F GRM > mean F GRM) and individuals that were less inbred than average (F GRM < mean F GRM). We did this by testing if the inclusion of a regression break point at the mean F GRM improved the models by comparing the resulting 9 candidate models using AICc.
RESULTS
Overall, the individual MS pedigree-based inbreeding coefficient (F PED) was not a good predictor of genomic estimators of inbreeding (Fig. S1a,c; Pearson’s r P = 0.05, n = 371), but its relationships with F GRM and F ROH were improved when including only individuals with at least two generations known (Fig. S1b,d; r P > 0.30, n = 59). F GRM and F ROH were strongly correlated (Fig. S1e,f; r P = 0.7, n = 371).
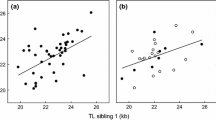
F PED varied from 0.000 to 0.250 (mean 0.007, 16.9% non-zero values). None of the models of F PED provided strong statistical support for a relationship with TL. The highest ranked model explaining variation in TL included a negative effect of F PED, but only slightly improved the fit of the baseline model (∆ 2:1 AICc = 0.8 [subscripts denote which ranked models are compared], w 1 = 0.36, ER 1 = w 1 /w 2 = 1.49, Table S1 in the supporting information). Thus, there was a tendency for TL to be shorter in more inbred sparrows (β F_PED = -0.169 ± 0.101, CI = [-0.366, 0.028], n = 1195, Fig. 1a; Table 2). The model ranked third (∆ 3:1 AICc = 1.3) indicated that TL was less associated with F PED in males than in females (β F_PED*sex[female] = -0.167 ± 0.196, CI = [-0.549, 0.216]), while the model ranked fourth (∆ 4 AICc = 1.9) indicated that TL was less associated with F PED in the Hestmannøy population than in the Træna population (β F_PED*island[Hestmannøy] = 0.115 ± 0.314, CI = [-0.498, 0.728]). However, due to high uncertainty in these parameter estimates, these effects are not deemed reliable.
Associations between early-life telomere length (log10-transformed) and various individual measures of inbreeding in wild house sparrows: (a) microsatellite pedigree-based inbreeding coefficient (F PED), (b) F PED for individuals with at least two full ancestral generations known, (c) testing for an interaction effect between F PED and first-year survival (survivors: n = 206 in grey, dotted regression line; non-survivors: n = 989 in black, solid regression line), (d) genomic inbreeding coefficient F GRM, (e) regression with a break point at the mean F GRM (0.016), and (f) runs-of-homozygosity F ROH. Black lines show the predicted effect of the inbreeding coefficient on TL from LMMs described in the text and the grey area shows 95% confidence intervals. Note that the y-axis is not scaled equally across panels and that color of points are graduated for visibility
When only including individuals with at least 2 full ancestral generations known (33.8% non-zero values), the model with F PED was ranked second (∆ 2:1 AICc = 1.1, β F_PED = -0.205 ± 0.198, CI = [-0.588, 0.189], n = 320, Fig. 1b, Table S2-3) and the baseline model was highest ranked.
There was a tendency for the negative effect of F PED on TL to be weaker in first-year survivors (n = 206, mean TL = 0.95 ± 0.02, mean F PED = 0.010 ± 0.003) than in non-survivors (n = 989, mean TL = 0.97 ± 0.01, mean F PED = 0.007 ± 0.001, β F_PED*first−year survival = 0.304 ± 0.201, CI = [-0.089, 0.697], n = 1195, Fig. 1c, Table S4). This effect was uncertain with a CI overlapping zero. This suggests that the following analyses using genomic estimators of inbreeding in recruits were not biased towards stronger inbreeding effects in recruits.
Genomic inbreeding coefficient (F GRM) estimates varied from -0.200 to 0.300 (mean 0.016, which is different from the expected 0, because F GRM was calculated across the whole metapopulation simultaneously, see Niskanen et al. 2020). The highest ranked model (∆ 2:1 AICc = 2.1, Table S5) showed that TL was shorter in more inbred sparrows (β F_GRM = -1.517 ± 0.293, CI = [-2.150, -0.920], n = 371, Fig. 1d, and Table 3). In addition, the effect of F GRM on TL was stronger in the Træna population (β F_GRM*island[Hestmannøy] = 0.824 ± 0.339, CI = [0.142, 1.529], Table 3) and in males (β F_GRM*sex[female] = 0.644 ± 0.314, CI = [0.034, 1.262], Table 3).
Including a break point at the mean F GRM improved the model compared to a model with no break point (comparing models without interaction terms which were ranked 8 and 5: ∆ 8:5 AICc = 4.5, see Table S6). The highest ranked model (∆ 2:1 AICc = 3.1, Table S6) revealed a strong negative association between TL and F GRM among individuals with F GRM < 0.016 but no association among inbred individuals with F GRM > 0.016 (Fig. 1e; Table 4). This indicates that a heterosis effect resulting in longer telomeres in outbred individuals may explain the negative association found between inbreeding and TL. This model also included an interaction term suggesting that this heterosis effect was stronger in the Træna population (Table 4).
The runs-of-homozygosity inbreeding coefficient (F ROH) estimates varied from 0.000 to 0.240 (mean 0.010, 73% non-zero values). The best model provided evidence for a negative effect of F ROH on TL (β F_ROH = -1.148 ± 0.512, CI = [-2.144, -0.153], n = 371, Fig. 1f, Table S7 and 5). This model also indicated that the negative effect of F ROH tended to be stronger in males (β F_ROH*sex [female] = 0.915 ± 0.610, CI = [-0.270, 2.102]).
DISCUSSION
We found evidence using genomic measures of inbreeding that more inbred house sparrow nestlings had shorter telomeres (Fig. 1). Individual differences in TL are established early in life (Entringer et al. 2018), are heritable (Dugdale and Richardson 2018; Pepke et al. 2021a), and are positively associated with fitness in some species (Heidinger et al. 2012; Wilbourn et al. 2018). Thus, short telomeres in more inbred individuals may underpin a physiological basis of inbreeding depression in fitness components that has been found in this species (Billing et al. 2012; Jensen et al. 2007; Niskanen et al. 2020) and in other wild animal populations (Keller and Waller 2002).
The effect of inbreeding on TL in house sparrows was negative across all measures of inbreeding, but only statistically significant (i.e. with confidence intervals not overlapping zero) when using genomic levels of inbreeding (Fig. 1d-f), probably because they are better at capturing homozygosity causing inbreeding depression compared to using a pedigree-based estimator (Fig. 1a-c, Alemu et al. 2021; Huisman et al. 2016; Kardos et al. 2016). For instance, the frequency peak at F PED=0 (see histograms in Fig. S1) is better resolved using F GRM, which is expected due to pedigree incompleteness and Mendelian sampling variation in realized inbreeding levels around the pedigree prediction (Huisman et al. 2016). Mating between full siblings or between parent and offspring (F = 0.25) resulted in a severe reduction in (relative) TL of 58% (F GRM), 48% (F ROH) or 11% (F PED) compared to breeding between unrelated individuals (Tables 2 and 3, and 5). However, such high levels of inbreeding were rare (Fig. 1), and our results may need to be confirmed using larger datasets of highly inbred individuals. TL may be under strong selection in natural populations (Voillemot et al. 2012). Consequently, strong inbreeding depression is expected for fitness components or traits that are under strong selection (Bérénos et al. 2016; DeRose and Roff 1999). The analyses using genomic estimators of inbreeding were limited to recruited individuals, but the negative effect of inbreeding on TL may be even stronger if very inbred individuals, presumably with short telomeres, do not survive their first year and were thus excluded from our analyses (Jensen et al. 2007; Wilbourn et al. 2018). There was a tendency for such an effect when using pedigree-based levels of inbreeding (Fig. 1c and Table S4).
We found some evidence that inbreeding had stronger negative effects on TL in males than females (Tables 3 and 5). Such sex-specific effects of inbreeding are known from other species de Boer et al. 2018a, b; Janicke et al. 2013), but have rarely been observed early in life. There was a weak tendency for longer TL in males than females (Tables 2, 3, 4 and 5), which has been observed in similar house sparrow populations (Pepke et al. 2021b). Thus, males may be better buffered against the effects of inbreeding on TL. However, no sex-specific differences in inbreeding depression were observed in adult sparrows across this study metapopulation (Niskanen et al. 2020).
Increased inbreeding may be accompanied by population decline in small populations (Bozzuto et al. 2019; Chen et al. 2016; Feng et al. 2019), which can drive populations to extinction (O’Grady et al. 2006; Saccheri et al. 1998; Wright et al. 2007; Niskanen et al. 2020) showed that inbreeding depression in adult sparrows in our study system varied little across years or across the different island environments inhabited by these house sparrows. Hence, the strength of inbreeding depression is similar between populations, but due to harboring more inbred individuals, the relative effect is stronger in smaller populations (Niskanen et al. 2020). Small declining populations may be characterized by gradual population-wide and trans-generational telomere erosion. For instance, Dupoué et al. (2017) observed shorter TL along an extinction risk gradient in populations of common lizards (Zootoca vivipara) that are disappearing from low altitudes at their southern range limit, presumably due to climate warming (Sinervo et al. 2010). Combined, these results suggest that TL may represent a potential physiological biomarker or molecular tool in conservation genetics addressing the viability of some small animal populations (Bebbington et al. 2016; Bergman et al. 2019; Dupoué et al. 2017; Madliger et al. 2020).
The negative effect of F GRM on TL (Fig. 1d) was stronger among individuals that were less related than the average population (Fig. 1e). This suggests that longer telomeres in outbred individuals may partly be attributed to a general heterosis effect (Charlesworth and Willis 2009) involving mating between immigrants and native individuals (Dickel et al. 2021; Ebert et al. 2002). In our study metapopulation, the proportion of dispersers among recruits can be high among the island populations (0.2 on average ranging from 0.0 to 1.0 across years and islands, Ranke et al. 2021; Saatoglu et al. 2021), and hence most islands are not strongly differentiated (Niskanen et al. 2020). We found that the negative effect of F GRM on TL was stronger in the Træna population (Tables 3 and 4). Træna is known to have a higher proportion of immigrants than Hestmannøy (Ranke et al. 2021), which may contribute to a stronger effect of heterosis in this population (Table 4). Furthermore, the gardens of Træna expose the sparrows to a different environment than the farms on Hestmannøy (Araya-Ajoy et al. 2019; Pärn et al. 2012). Inbreeding depression is expected to have more severe consequences under environmental stress (Armbruster and Reed 2005; Reed et al. 2002), such as harsh weather or competition de Boer et al. 2018a; Fox and Reed 2011; Marr et al. 2006). Telomeres shorten due to environmental stressors such as harsh abiotic conditions (Chatelain et al. 2020). We speculate that environmental differences between the habitats of the two sparrow populations may explain the exacerbated effects of inbreeding on TL in the Træna population. For instance, in juvenile Seychelles warblers a negative relationship between homozygosity and TL was found only in poor seasons, i.e. when food availability was low (Bebbington et al. 2016). In adult Seychelles warblers, the effect of homozygosity on TL was consistently negative across seasons, suggesting that the negative effects of inbreeding accumulate through life and are reflected in telomere erosion (Bebbington et al. 2016). Here, we showed that inbreeding manifests in TL already at the nestling stage in a similar wild passerine.
We measured TL in blood, thus it is possible that inbreeding or heterosis only affected telomeres in erythrocytes (Manning et al. 2002; Olsson et al. 2020). However, this is unlikely because TLs often correlate well across tissues within the organism (Daniali et al. 2013; Demanelis et al. 2020; Reichert et al. 2013), especially in early-life (Prowse and Greider 1995). Although genomic inbreeding estimates were only available for first-year survivors, we may have avoided confounding effects of selective mortality of inbred individuals at much older ages by measuring TL already at the nestling stage (Hemmings et al. 2012; Sánchez-Montes et al. 2020). Furthermore, since the mutation accumulation theory of senescence predicts that deleterious effects of inbreeding increase with age (Charlesworth and Hughes 1996; Keller et al. 2008), we may expect that the effect on TL is persistent and potentially stronger in adult sparrows. Thus, future studies are required to investigate if inbreeding leads to persistently eroded TL throughout life, and if there are combined fitness consequences of any interaction between TL and inbreeding in wild populations. Even in the absence of a mechanism directly linking inbreeding and TL via the effects of oxidative stress (cf. the introduction), we may find inbred individuals to have short telomeres, because inbreeding impairs other physiological processes that affects both fitness and TL (Bebbington et al. 2016). Thus, the conflicting evidence in the literature of an effect of inbreeding on TL (reviewed in the introduction) suggests that an experimental procedure is needed to further elucidate the mechanisms underlying the correlation reported here (Manning et al. 2002), especially in wild populations.
In conclusion, the negative associations between inbreeding levels and TL found in this study suggest that TL may reveal subtle somatic costs of inbreeding in wild populations, and thereby demonstrates a potential route by which inbreeding negatively impacts the physiological state of an organism in early life. The observation of a potential heterosis effect on TL suggests that maintenance of dispersal within this metapopulation is important for mitigating the negative effects of inbreeding.
Availability of data and material
Data is available on the Open Science Framework (OSF) doi:https://doi.org/10.17605/OSF.IO/VN8GE.
Code Availability
Not applicable.
References
Akaike H (1973) Information theory and an extension of the maximum likelihood principle. Paper presented at the Second International Symposium on Information Theory, Akademiai Kiado, Budapest
Alemu SW, Kadri NK, Harland C, Faux P, Charlier C, Caballero A, Druet T (2021) An evaluation of inbreeding measures using a whole-genome sequenced cattle pedigree. Heredity 126(3):410–423. doi:https://doi.org/10.1038/s41437-020-00383-9
Angelier F, Vleck CM, Holberton RL, Marra PP (2013) Telomere length, non-breeding habitat and return rate in male American redstarts. Funct Ecol 27(2):342–350. doi:https://doi.org/10.1111/1365-2435.12041
Araya-Ajoy YG, Ranke PS, Kvalnes T, Rønning B, Holand H, Myhre AM, Pärn H, Jensen H, Ringsby TH, Sæther B-E, Wright J (2019) Characterizing morphological (co)variation using structural equation models: Body size, allometric relationships and evolvability in a house sparrow metapopulation. Evolution 73(3):452–466. doi:https://doi.org/10.1111/evo.13668
Araya-Ajoy YG, Niskanen AK, Froy H, Ranke PS, Kvalnes T, Rønning B, Pepke ML, Jensen H, Ringsby TH, Sæther B-E, Wright J (2021) Variation in generation time reveals density regulation as an important driver of pace-of-life in a bird metapopulation. Ecol Lett 24:2077–2087. doi:https://doi.org/10.1111/ele.13835
Armbruster P, Reed DH (2005) Inbreeding depression in benign and stressful environments. Heredity 95(3):235–242. doi:https://doi.org/10.1038/sj.hdy.6800721
Barnes RP, Fouquerel E, Opresko PL (2019) The impact of oxidative DNA damage and stress on telomere homeostasis. Mech Ageing Dev 177:37–45. doi:https://doi.org/10.1016/j.mad.2018.03.013
Barrett EL, Richardson DS (2011) Sex differences in telomeres and lifespan. Aging Cell 10(6):913–921. doi:https://doi.org/10.1111/j.1474-9726.2011.00741.x
Barrett ELB, Burke TA, Hammers M, Komdeur J, Richardson DS (2013) Telomere length and dynamics predict mortality in a wild longitudinal study. Mol Ecol 22(1):249–259. doi:https://doi.org/10.1111/mec.12110
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48. doi:https://doi.org/10.18637/jss.v067.i01
Bateson M (2016) Cumulative stress in research animals: Telomere attrition as a biomarker in a welfare context? BioEssays 38(2):201–212. doi:https://doi.org/10.1002/bies.201500127
Bateson M, Poirier C (2019) Can biomarkers of biological age be used to assess cumulative lifetime experience? Anim Welf 28(1):41–56. doi:https://doi.org/10.7120/09627286.28.1.041
Bebbington K, Spurgin LG, Fairfield EA, Dugdale HL, Komdeur J, Burke T, Richardson DS (2016) Telomere length reveals cumulative individual and transgenerational inbreeding effects in a passerine bird. Mol Ecol 25(12):2949–2960. doi:https://doi.org/10.1111/mec.13670
Becker PJ, Reichert S, Zahn S, Hegelbach J, Massemin S, Keller LF, Postma E, Criscuolo F (2015) Mother-offspring and nest-mate resemblance but no heritability in early-life telomere length in white-throated dippers. Proc Biol Sci 282(1807):20142924. doi:https://doi.org/10.1098/rspb.2014.2924
Benton CH, Delahay RJ, Smith FAP, Robertson A, McDonald RA, Young AJ, Burke TA, Hodgson D (2018) Inbreeding intensifies sex- and age-dependent disease in a wild mammal. J Anim Ecol 87(6):1500–1511. doi:https://doi.org/10.1111/1365-2656.12878
Bérénos C, Ellis PA, Pilkington JG, Pemberton JM (2016) Genomic analysis reveals depression due to both individual and maternal inbreeding in a free-living mammal population. Mol Ecol 25(13):3152–3168. doi:https://doi.org/10.1111/mec.13681
Bergman JN, Bennett JR, Binley AD, Cooke SJ, Fyson V, Hlina BL, Reid CH, Vala MA, Madliger CL (2019) Scaling from individual physiological measures to population-level demographic change: Case studies and future directions for conservation management. Biol Conserv 238:108242. doi:https://doi.org/10.1016/j.biocon.2019.108242
Billing AM, Lee AM, Skjelseth S, Borg AA, Hale MC, Slate J, Parn H, Ringsby TH, Saether BE, Jensen H (2012) Evidence of inbreeding depression but not inbreeding avoidance in a natural house sparrow population. Mol Ecol 21(6):1487–1499. doi:https://doi.org/10.1111/j.1365-294X.2012.05490.x
Blackburn EH, Gall JG (1978) A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol 120(1):33–53. doi:https://doi.org/10.1016/0022-2836(78)90294-2
Boonekamp JJ, Simons MJ, Hemerik L, Verhulst S (2013) Telomere length behaves as biomarker of somatic redundancy rather than biological age. Aging Cell 12(2):330–332. doi:https://doi.org/10.1111/acel.12050
Bosse M, Megens H-J, Derks MFL, de Cara ÁMR, Groenen MAM (2018) Deleterious alleles in the context of domestication, inbreeding, and selection. Evol Appl 12(1):6–17. doi:https://doi.org/10.1111/eva.12691
Bozzuto C, Biebach I, Muff S, Ives AR, Keller LF (2019) Inbreeding reduces long-term growth of Alpine ibex populations. Nat Ecol Evol 3(9):1359–1364. doi:https://doi.org/10.1038/s41559-019-0968-1
Burnham KP, Anderson DR (2002) Model selection and multimodel inference. A practical information-theoretic approach, 2 edn. Springer-Verlag, New York, U.S.A.
Cawthon RM (2002) Telomere measurement by quantitative PCR. Nucleic Acids Res 30(10):e47. doi:https://doi.org/10.1093/nar/30.10.e47
Červenák F, Sepšiová R, Nosek J, Tomáška Ľ (2021) Step-by-step evolution of telomeres: Lessons from yeasts. Genome Biol Evol 13(2), evaa268. doi:https://doi.org/10.1093/gbe/evaa268
Charlesworth B, Hughes KA (1996) Age-specific inbreeding depression and components of genetic variance in relation to the evolution of senescence. Proc Natl Acad Sci USA 93(12):6140–6145. doi:https://doi.org/10.1073/pnas.93.12.6140
Charlesworth D, Willis JH (2009) The genetics of inbreeding depression. Nat Rev Genet 10(11):783–796. doi:https://doi.org/10.1038/nrg2664
Charpentier MJE, Williams CV, Drea CM (2008) Inbreeding depression in ring-tailed lemurs (Lemur catta): genetic diversity predicts parasitism, immunocompetence, and survivorship. Conserv Genet 9(6):1605–1615. doi:https://doi.org/10.1007/s10592-007-9499-4
Chatelain M, Drobniak SM, Szulkin M (2020) The association between stressors and telomeres in non-human vertebrates: a meta-analysis. Ecol Lett 23(2):381–398. doi:https://doi.org/10.1111/ele.13426
Chen N, Cosgrove EJ, Bowman R, Fitzpatrick JW, Clark AG (2016) Genomic consequences of population decline in the endangered Florida scrub-jay. Curr Biol 26(21):2974–2979. doi:https://doi.org/10.1016/j.cub.2016.08.062
Coster A (2012) pedigree: Pedigree functions. R package version 1.4. https://CRAN.R-project.org/package=pedigree
Criscuolo F, Bize P, Nasir L, Metcalfe NB, Foote CG, Griffiths K, Gault EA, Monaghan P (2009) Real-time quantitative PCR assay for measurement of avian telomeres. J Avian Biol 40(3):342–347. doi:https://doi.org/10.1111/j.1600-048X.2008.04623.x
Curik I, Ferenčaković M, Sölkner J (2014) Inbreeding and runs of homozygosity: A possible solution to an old problem. Livest Sci 166:26–34. doi:https://doi.org/10.1016/j.livsci.2014.05.034
Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, Desai K, Granick M, Aviv A (2013) Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun 4:1597. doi:https://doi.org/10.1038/ncomms2602
de Boer RA, Costantini D, Casasole G, AbdElgawad H, Asard H, Eens M, Müller W (2018a) Sex-specific effects of inbreeding and early life conditions on the adult oxidative balance. Curr Zool 64(5):631–639. doi:https://doi.org/10.1093/cz/zox076
de Boer RA, Eens M, Müller W (2018b) Sex-specific effects of inbreeding on reproductive senescence. Proceedings of the Royal Society B: Biological Sciences, 285(1879), 20180231. doi:https://doi.org/10.1098/rspb.2018.0231
Demanelis K, Jasmine F, Chen LS, Chernoff M, Tong L, Delgado D, Zhang C, Shinkle J, Sabarinathan M, Lin H, Ramirez E, Oliva M, Kim-Hellmuth S, Stranger BE, Lai T-P, Aviv A, Ardlie KG, Aguet F, Ahsan H, Doherty JA, Kibriya MG, Pierce BL (2020) Determinants of telomere length across human tissues. Science 369(6509):eaaz6876. doi:https://doi.org/10.1126/science.aaz6876
DeRose MA, Roff DA (1999) A comparison of inbreeding depression in life-history and morphological traits in animals. Evolution 53(4):1288–1292. doi:https://doi.org/10.1111/j.1558-5646.1999.tb04541.x
Dickel L, Arcese P, Nietlisbach P, Keller LF, Jensen H, Reid JM (2021) Are immigrants outbred and unrelated? Testing standard assumptions in a wild metapopulation. Mol Ecol. doi:https://doi.org/10.1111/mec.16173
Dugdale HL, Richardson DS (2018) Heritability of telomere variation: it is all about the environment! Philos Trans R Soc Lond B Biol Sci 373(1741):20160450. doi:https://doi.org/10.1098/rstb.2016.0450
Dupoué A, Rutschmann A, Le Galliard JF, Clobert J, Angelier F, Marciau C, Ruault S, Miles D, Meylan S (2017) Shorter telomeres precede population extinction in wild lizards. Sci Rep 7(1):16976. doi:https://doi.org/10.1038/s41598-017-17323-z
Ebert D, Haag C, Kirkpatrick M, Riek M, Jürgen H, Pajunen VI (2002) A selective advantage to immigrant genes in a Daphnia metapopulation. Science 295(5554):485–488. doi:https://doi.org/10.1126/science.1067485
Eisenberg DT (2011) An evolutionary review of human telomere biology: the thrifty telomere hypothesis and notes on potential adaptive paternal effects. Am J Hum Biol 23(2):149–167. doi:https://doi.org/10.1002/ajhb.21127
Elgvin TO, Trier CN, Tørresen OK, Hagen IJ, Lien S, Nederbragt AJ, Ravinet M, Jensen H, Sætre G-P (2017) The genomic mosaicism of hybrid speciation. Sci Adv 3(6):e1602996–e1602996. doi:https://doi.org/10.1126/sciadv.1602996
Entringer S, de Punder K, Buss C, Wadhwa PD (2018) The fetal programming of telomere biology hypothesis: an update. Philosophical Trans Royal Soc B: Biol Sci 373(1741):20170151. doi:https://doi.org/10.1098/rstb.2017.0151
Feng S, Fang Q, Barnett R, Li C, Han S, Kuhlwilm M, Zhou L, Pan H, Deng Y, Chen G, Gamauf A, Woog F, Prys-Jones R, Marques-Bonet T, Gilbert MTP, Zhang G (2019) The genomic footprints of the fall and recovery of the crested ibis. Curr Biol 29(2):340–349e347. doi:https://doi.org/10.1016/j.cub.2018.12.008
Forsyth NR, Elder FFB, Shay JW, Wright WE (2005) Lagomorphs (rabbits, pikas and hares) do not use telomere-directed replicative aging in vitro. Mech Ageing Dev 126(6):685–691. doi:https://doi.org/10.1016/j.mad.2005.01.003
Fox CW, Reed DH (2011) Inbreeding depression increases with environmental stress: An experimental study and meta-analysis. Evolution 65(1):246–258. doi:https://doi.org/10.1111/j.1558-5646.2010.01108.x
Frankham R (2015) Genetic rescue of small inbred populations: meta-analysis reveals large and consistent benefits of gene flow. Mol Ecol 24(11):2610–2618. doi:https://doi.org/10.1111/mec.13139
Froy H, Underwood SL, Dorrens J, Seeker LA, Watt K, Wilbourn RV, Pilkington JG, Harrington L, Pemberton JM, Nussey DH (2021) Heritable variation in telomere length predicts mortality in Soay sheep. Proceedings of the National Academy of Sciences, 118(15), e2020563118. doi:https://doi.org/10.1073/pnas.2020563118
Hadfield J (2010) MCMC methods for multi-response generalized linear mixed models: The MCMCglmm R package. J Stat Softw 1(2):1–22. doi:https://doi.org/10.18637/jss.v033.i02
Hagen IJ, Lien S, Billing AM, Elgvin TO, Trier C, Niskanen AK, Tarka M, Slate J, Sætre GP, Jensen H (2020) A genome-wide linkage map for the house sparrow (Passer domesticus) provides insights into the evolutionary history of the avian genome. Mol Ecol Resour 20(2):544–559. doi:https://doi.org/10.1111/1755-0998.13134
Hall ME, Nasir L, Daunt F, Gault EA, Croxall JP, Wanless S, Monaghan P (2004) Telomere loss in relation to age and early environment in long-lived birds. Proc Biol Sci 271(1548):1571–1576. doi:https://doi.org/10.1098/rspb.2004.2768
Hanson HE, Mathews NS, Hauber ME, Martin LB (2020) The house sparrow in the service of basic and applied biology. Elife 9:e52803. doi:https://doi.org/10.7554/eLife.52803
Harley CB, Futcher AB, Greider CW (1990) Telomeres shorten during ageing of human fibroblasts. Nature 345(6274):458–460. doi:https://doi.org/10.1038/345458a0
Harrisson KA, Magrath MJL, Yen JDL, Pavlova A, Murray N, Quin B, Menkhorst P, Miller KA, Cartwright K, Sunnucks P (2019) Lifetime fitness costs of inbreeding and being inbred in a critically endangered bird. Curr Biol 29(16):2711–2717e2714. doi:https://doi.org/10.1016/j.cub.2019.06.064
Haussmann MF, Marchetto NM (2010) Telomeres: Linking stress and survival, ecology and evolution. Curr Zool 56(6):714–727
Hedrick PW, Kalinowski ST (2000) Inbreeding depression in conservation biology. Annu Rev Ecol Syst 31(1):139–162. doi:https://doi.org/10.1146/annurev.ecolsys.31.1.139
Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P (2012) Telomere length in early life predicts lifespan. Proc Natl Acad Sci U S A 109(5):1743–1748. doi:https://doi.org/10.1073/pnas.1113306109
Heidinger BJ, Kucera AC, Kittilson JD, Westneat DF (2021) Longer telomeres during early life predict higher lifetime reproductive success in females but not males. Proceedings of the Royal Society B: Biological Sciences, 288(1951), 20210560. doi:https://doi.org/10.1098/rspb.2021.0560
Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J (2007) qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8(2):R19. doi:https://doi.org/10.1186/gb-2007-8-2-r19
Hemann MT, Greider CW (2000) Wild-derived inbred mouse strains have short telomeres. Nucleic Acids Res 28(22):4474–4478. doi:https://doi.org/10.1093/nar/28.22.4474
Hemmings NL, Slate J, Birkhead TR (2012) Inbreeding causes early death in a passerine bird. Nat Commun 3(1):863. doi:https://doi.org/10.1038/ncomms1870
Huisman J, Kruuk LEB, Ellis PA, Clutton-Brock T, Pemberton JM (2016) Inbreeding depression across the lifespan in a wild mammal population. Proceedings of the National Academy of Sciences, 201518046. doi:https://doi.org/10.1073/pnas.1518046113
Hurvich CM, Tsai C-L (1989) Regression and time series model selection in small samples. Biometrika 76(2):297–307. doi:https://doi.org/10.1093/biomet/76.2.297
Janicke T, Vellnow N, Sarda V, David P (2013) Sex-specific inbreeding depression depends on the strength of male-male competition. Evolution 67(10):2861–2875. doi:https://doi.org/10.1111/evo.12167
Jensen H, Saether BE, Ringsby TH, Tufto J, Griffith SC, Ellegren H (2003) Sexual variation in heritability and genetic correlations of morphological traits in house sparrow (Passer domesticus). J Evol Biol 16(6):1296–1307
Jensen H, Bremset EM, Ringsby TH, Sæther BE (2007) Multilocus heterozygosity and inbreeding depression in an insular house sparrow metapopulation. Mol Ecol 16(19):4066–4078. doi:https://doi.org/10.1111/j.1365-294X.2007.03452.x
Jensen H, Steinsland I, Ringsby TH, Sæther BE (2008) Evolutionary dynamics of a sexual ornament in the house sparrow (Passer domesticus): The role of indirect selection within and between sexes. Evolution 62(6):1275–1293. doi:https://doi.org/10.1111/j.1558-5646.2008.00395.x
Kalinowski ST, Taper ML, Marshall TC (2007) Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol 16(5):1099–1106. doi:https://doi.org/10.1111/j.1365-294X.2007.03089.x
Kardos M, Taylor HR, Ellegren H, Luikart G, Allendorf FW (2016) Genomics advances the study of inbreeding depression in the wild. Evol Appl 9(10):1205–1218. doi:https://doi.org/10.1111/eva.12414
Kawanishi S, Oikawa S (2004) Mechanism of telomere shortening by oxidative stress. Ann N Y Acad Sci 1019(1):278–284. doi:https://doi.org/10.1196/annals.1297.047
Keller LF, Waller DM (2002) Inbreeding effects in wild populations. Trends Ecol Evol 17(5):230–241. doi:https://doi.org/10.1016/S0169-5347(02)02489-8
Keller LF, Reid JM, Arcese P (2008) Testing evolutionary models of senescence in a natural population: age and inbreeding effects on fitness components in song sparrows. Proceedings of the Royal Society B: Biological Sciences, 275(1635), 597–604. doi:https://doi.org/10.1098/rspb.2007.0961
Ketola T, Kotiaho JS (2009) Inbreeding, energy use and condition. J Evol Biol 22(4):770–781. doi:https://doi.org/10.1111/j.1420-9101.2009.01689.x
Kristensen TN, Sørensen P, Kruhøffer M, Pedersen KS, Loeschcke V (2005) Genome-wide analysis on inbreeding effects on gene expression in Drosophila melanogaster. Genetics 171(1):157–167. doi:https://doi.org/10.1534/genetics.104.039610
Kristensen TN, Pedersen KS, Vermeulen CJ, Loeschcke V (2010) Research on inbreeding in the ‘omic’ era. Trends Ecol Evol 25(1):44–52. doi:https://doi.org/10.1016/j.tree.2009.06.014
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153(6):1194–1217. doi:https://doi.org/10.1016/j.cell.2013.05.039
Losdat S, Arcese P, Sampson L, Villar N, Reid JM (2016) Additive genetic variance and effects of inbreeding, sex and age on heterophil to lymphocyte ratio in song sparrows. Funct Ecol 30(7):1185–1195. doi:https://doi.org/10.1111/1365-2435.12586
Lundregan SL, Hagen IJ, Gohli J, Niskanen AK, Kemppainen P, Ringsby TH, Kvalnes T, Pärn H, Rønning B, Holand H, Ranke PS, Båtnes AS, Selvik L-K, Lien S, Sæther B-E, Husby A, Jensen H (2018) Inferences of genetic architecture of bill morphology in house sparrow using a high-density SNP array point to a polygenic basis. Mol Ecol 27(17):3498–3514. doi:https://doi.org/10.1111/mec.14811
Madliger CL, Franklin CE, Love OP, Cooke SJ (2020) Conservation physiology: Applications for wildlife conservation and management. Oxford University Press, USA
Manning EL, Crossland J, Dewey MJ, Van Zant G (2002) Influences of inbreeding and genetics on telomere length in mice. Mamm Genome 13(5):234–238. doi:https://doi.org/10.1007/s003350020027
Mansour H, Chowdari K, Fathi W, Elassy M, Ibrahim I, Wood J, Bamne M, Tobar S, Yassin A, Salah H, Elsayed H, Eissa A, El-Boraie H, Ibrahim NE, Elsayed M, El-Bahaei W, Gomaa Z, El-Chennawi F, Nimgaonkar VL (2011) Does telomere length mediate associations between inbreeding and increased risk for bipolar I disorder and schizophrenia? Psychiatry Res 188(1):129–132. doi:https://doi.org/10.1016/j.psychres.2011.01.010
Marr AB, Arcese P, Hochachka WM, Reid JM, Keller LF (2006) Interactive effects of environmental stress and inbreeding on reproductive traits in a wild bird population. J Anim Ecol 75(6):1406–1415. doi:https://doi.org/10.1111/j.1365-2656.2006.01165.x
McLennan D, Auer SK, McKelvey S, McKelvey L, Anderson G, Boner W, Duprez JS, Metcalfe NB (2021) Habitat restoration weakens negative environmental effects on telomere dynamics. Mol Ecol. doi:https://doi.org/10.1111/mec.15980
McQuillan R, Leutenegger A-L, Abdel-Rahman R, Franklin CS, Pericic M, Barac-Lauc L, Smolej-Narancic N, Janicijevic B, Polasek O, Tenesa A, MacLeod AK, Farrington SM, Rudan P, Hayward C, Vitart V, Rudan I, Wild SH, Dunlop MG, Wright AF, Campbell H, Wilson JF (2008) Runs of homozygosity in European populations. Am J Hum Genet 83(3):359–372. doi:https://doi.org/10.1016/j.ajhg.2008.08.007
Monaghan P (2014) Organismal stress, telomeres and life histories. J Exp Biol 217(Pt 1):57–66. doi:https://doi.org/10.1242/jeb.090043
Monaghan P, Ozanne SE (2018) Somatic growth and telomere dynamics in vertebrates: relationships, mechanisms and consequences. Philos Trans R Soc Lond B Biol Sci 373(1741):20160446. doi:https://doi.org/10.1098/rstb.2016.0446
Moyers BT, Morrell PL, McKay JK (2018) Genetic costs of domestication and improvement. J Hered 109(2):103–116. doi:https://doi.org/10.1093/jhered/esx069
Niskanen AK, Billing AM, Holand H, Hagen IJ, Araya-Ajoy YG, Husby A, Rønning B, Myhre AM, Ranke PS, Kvalnes T, Pärn H, Ringsby TH, Lien S, Sæther B-E, Muff S, Jensen H (2020) Consistent scaling of inbreeding depression in space and time in a house sparrow metapopulation. Proceedings of the National Academy of Sciences, 117(25), 14584. doi:https://doi.org/10.1073/pnas.1909599117
Nuzhdin SV, Reiwitch SG (2002) Heterosis of quantitative trait loci affecting lifespan in Drosophila melanogaster. Russian J Genet 38(7):766–770. doi:https://doi.org/10.1023/A:1016335504009
O’Grady JJ, Brook BW, Reed DH, Ballou JD, Tonkyn DW, Frankham R (2006) Realistic levels of inbreeding depression strongly affect extinction risk in wild populations. Biol Conserv 133(1):42–51. doi:https://doi.org/10.1016/j.biocon.2006.05.016
O’Hare TH, Delany ME (2009) Genetic variation exists for telomeric array organization within and among the genomes of normal, immortalized, and transformed chicken systems. Chromosome Res 17(8):947. doi:https://doi.org/10.1007/s10577-009-9082-6
Okada K, Blount JD, Sharma MD, Snook RR, Hosken DJ (2011) Male attractiveness, fertility and susceptibility to oxidative stress are influenced by inbreeding in Drosophila simulans. J Evol Biol 24(2):363–371. doi:https://doi.org/10.1111/j.1420-9101.2010.02170.x
Olsson M, Wapstra E, Friesen CR (2018) Evolutionary ecology of telomeres: a review. Ann N Y Acad Sci 1422(1):5–28. doi:https://doi.org/10.1111/nyas.13443
Olsson M, Geraghty NJ, Wapstra E, Wilson M (2020) Telomere length varies substantially between blood cell types in a reptile. R Soc Open Sci 7(6):192136. doi:https://doi.org/10.1098/rsos.192136
Ozawa Y, Watanabe K, Toda T, Shibuya S, Okumura N, Okamoto N, Sato Y, Kawashima I, Kawamura K, Shimizu T (2019) Heterosis extends the reproductive ability in aged female mice. Biol Reprod 100(4):1082–1089. doi:https://doi.org/10.1093/biolre/ioy260
Pärn H, Ringsby TH, Jensen H, Sæther B-E (2012) Spatial heterogeneity in the effects of climate and density-dependence on dispersal in a house sparrow metapopulation. Proceedings of the Royal Society B: Biological Sciences, 279(1726), 144–152. doi:https://doi.org/10.1098/rspb.2011.0673
Pedersen KS, Kristensen TN, Loeschcke V, Petersen BO, Duus J, Nielsen NC, Malmendal A (2008) Metabolomic signatures of inbreeding at benign and stressful temperatures in Drosophila melanogaster. Genetics 180(2):1233–1243. doi:https://doi.org/10.1534/genetics.108.089144
Pepke ML, Eisenberg DTA (2021) On the comparative biology of mammalian telomeres: Telomere length co-evolves with body mass, lifespan and cancer risk. Mol Ecol. doi:https://doi.org/10.1111/mec.15870
Pepke ML, Kvalnes T, Lundregan SL, Boner W, Monaghan P, Sæther B-E, Jensen H, Ringsby TH (2021a) Genetic architecture and heritability of early-life telomere length in a wild passerine. Mol Ecol. doi:https://doi.org/10.1111/mec.16288
Pepke ML, Kvalnes T, Rønning B, Jensen H, Boner W, Sæther B-E, Monaghan P, Ringsby TH (2021b) Artificial size selection experiment reveals telomere length dynamics and fitness consequences in a wild passerine. Mol Ecol. doi:https://doi.org/10.1111/mec.16340
Pepke ML, Ringsby TH, Eisenberg DTA (2021c) Early-life telomere length covaries with life-history traits and scales with chromosome length in birds. bioRxiv, 2021.2008.2007.455497. doi:https://doi.org/10.1101/2021.08.07.455497
Pepper GV, Bateson M, Nettle D (2018) Telomeres as integrative markers of exposure to stress and adversity: a systematic review and meta-analysis. R Soc Open Sci 5(8):180744. doi:https://doi.org/10.1098/rsos.180744
Prowse KR, Greider CW (1995) Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proceedings of the National Academy of Sciences, 92(11), 4818. doi:https://doi.org/10.1073/pnas.92.11.4818
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, Sham PC (2007) PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81(3):559–575. doi:https://doi.org/10.1086/519795
R Core Team (2020) R: A language and environment for statistical computing. (Version 3.6.3). Vienna, Austria. R Foundation for Statistical Computing, Retrieved from www.R-project.org/
Ranke PS, Araya-Ajoy YG, Ringsby TH, Pärn H, Rønning B, Jensen H, Wright J, Sæther B-E (2021) Spatial structure and dispersal dynamics in a house sparrow metapopulation. J Anim Ecol. doi:https://doi.org/10.1111/1365-2656.13580
Ravinet M, Elgvin Tore O, Trier C, Aliabadian M, Gavrilov A, Sætre G-P (2018) Signatures of human-commensalism in the house sparrow genome. Proceedings of the Royal Society B: Biological Sciences, 285(1884), 20181246. doi:https://doi.org/10.1098/rspb.2018.1246
Reed DH, Briscoe DA, Frankham R (2002) Inbreeding and extinction: The effect of environmental stress and lineage. Conserv Genet 3(3):301–307. doi:https://doi.org/10.1023/A:1019948130263
Reichert S, Criscuolo F, Verinaud E, Zahn S, Massemin S (2013) Telomere length correlations among somatic tissues in adult zebra finches. PLoS ONE 8(12):e81496. doi:https://doi.org/10.1371/journal.pone.0081496
Reichert S, Stier A (2017) Does oxidative stress shorten telomeres in vivo? A review. Biol Lett 13(12):20170463. doi:https://doi.org/10.1098/rsbl.2017.0463
Reid JM, Arcese P, Keller LF (2003) Inbreeding depresses immune response in song sparrows (Melospiza melodia): direct and inter–generational effects. Proceedings of the Royal Society of London. Series B: Biological Sciences, 270(1529), 2151–2157. doi:https://doi.org/10.1098/rspb.2003.2480
Remot F, Ronget V, Froy H, Rey B, Gaillard JM, Nussey DH, Lemaitre JF (2020) No sex differences in adult telomere length across vertebrates: a meta-analysis. R Soc Open Sci 7(11):200548. doi:https://doi.org/10.1098/rsos.200548
Remot F, Ronget V, Froy H, Rey B, Gaillard J-M, Nussey DH, Lemaître J-F (2021) Decline in telomere length with increasing age across non-human vertebrates: a meta-analysis. Mol Ecol. doi:https://doi.org/10.1111/mec.16145
Ringsby TH, Sæther B-E, Jensen H, Engen S (2006) Demographic characteristics of extinction in a small, insular population of house sparrows in northern norway. Conserv Biol 20(6):1761–1767. doi:https://doi.org/10.1111/j.1523-1739.2006.00568.x
Ringsby TH, Jensen H, Pärn H, Kvalnes T, Boner W, Gillespie R, Holand H, Hagen IJ, Rønning B, Sæther BE, Monaghan P (2015) On being the right size: increased body size is associated with reduced telomere length under natural conditions. Proc Biol Sci 282(1820):20152331. doi:https://doi.org/10.1098/rspb.2015.2331
Rising JD, Somers KM (1989) The measurement of overall body size in birds. Auk 106(4):666–674
Saatoglu D, Niskanen AK, Kuismin M, Ranke PS, Hagen IJ, Araya-Ajoy YG, Kvalnes T, Pärn H, Rønning B, Ringsby TH, Sæther B-E, Husby A, Sillanpää MJ, Jensen H (2021) Dispersal in a house sparrow metapopulation: An integrative case study of genetic assignment calibrated with ecological data and pedigree information. Mol Ecol. doi:https://doi.org/10.1111/mec.16083
Saccheri I, Kuussaari M, Kankare M, Vikman P, Fortelius W, Hanski I (1998) Inbreeding and extinction in a butterfly metapopulation. Nature 392(6675):491–494. doi:https://doi.org/10.1038/33136
Sánchez-Montes G, Martínez-Solano Í, Díaz-Paniagua C, Vilches A, Ariño AH, Gomez-Mestre I (2020) Telomere attrition with age in a wild amphibian population. Biol Lett 16(7):20200168. doi:https://doi.org/10.1098/rsbl.2020.0168
Seluanov A, Hine C, Bozzella M, Hall A, Sasahara THC, Ribeiro AACM, Catania KC, Presgraves DC, Gorbunova V (2008) Distinct tumor suppressor mechanisms evolve in rodent species that differ in size and lifespan. Aging Cell 7(6):813–823. doi:https://doi.org/10.1111/j.1474-9726.2008.00431.x
Senar J, Pascual J (1997) Keel and tarsus length may provide a good predictor of avian body size. Ardea 85:269–274
Simons MJ (2015) Questioning causal involvement of telomeres in aging. Ageing Res Rev 24(Pt):191–196. doi:https://doi.org/10.1016/j.arr.2015.08.002
Sinervo B, Méndez-de-la-Cruz F, Miles DB, Heulin B, Bastiaans E, Villagrán-Santa Cruz M, Lara-Resendiz R, Martínez-Méndez N, Calderón-Espinosa ML, Meza-Lázaro RN, Gadsden H, Avila LJ, Morando M, De la Riva IJ, Sepulveda PV, Rocha CFD, Ibargüengoytía N, Puntriano CA, Massot M, Lepetz V, Oksanen TA, Chapple DG, Bauer AM, Branch WR, Clobert J, Sites JW (2010) Erosion of lizard diversity by climate change and altered thermal niches. Science 328(5980):894. doi:https://doi.org/10.1126/science.1184695
Sridevi V, Uma KD, Sivaramakrishnan S, Isola NR (2002) Telomere length as related to chromosome length in the genus Pennisetum. Cytologia 67(2):185–190
Sudyka J, Arct A, Drobniak SM, Gustafsson L, Cichoń M (2019) Birds with high lifetime reproductive success experience increased telomere loss. Biol Lett 15(1):20180637. doi:https://doi.org/10.1098/rsbl.2018.0637
Szulkin M, Sheldon BC (2007) The environmental dependence of inbreeding depression in a wild bird population. PLoS ONE 2(10):e1027. doi:https://doi.org/10.1371/journal.pone.0001027
Tricola GM, Simons MJP, Atema E, Boughton RK, Brown JL, Dearborn DC, Divoky G, Eimes JA, Huntington CE, Kitaysky AS, Juola FA, Lank DB, Litwa HP, Mulder EGA, Nisbet ICT, Okanoya K, Safran RJ, Schoech SJ, Schreiber EA, Thompson PM, Verhulst S, Wheelwright NT, Winkler DW, Young R, Vleck CM, Haussmann MF (2018) The rate of telomere loss is related to maximum lifespan in birds. Philosophical Trans Royal Soc B: Biol Sci 373(1741):20160445. doi:https://doi.org/10.1098/rstb.2016.0445
Voillemot M, Hine K, Zahn S, Criscuolo F, Gustafsson L, Doligez B, Bize P (2012) Effects of brood size manipulation and common origin on phenotype and telomere length in nestling collared flycatchers. BMC Ecol 12(1):17. doi:https://doi.org/10.1186/1472-6785-12-17
von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27(7):339–344
Wang J (2014) Marker-based estimates of relatedness and inbreeding coefficients: an assessment of current methods. J Evol Biol 27(3):518–530. doi:https://doi.org/10.1111/jeb.12315
Weinstein BS, Ciszek D (2002) The reserve-capacity hypothesis: evolutionary origins and modern implications of the trade-off between tumor-suppression and tissue-repair. Exp Gerontol 37(5):615–627. doi:https://doi.org/10.1016/S0531-5565(02)00012-8
Wellmann R (2021) optiSel: Optimum Contribution Selection and Population Genetics. R package version 2.0.5. Retrieved from https://CRAN.R-project.org/package=optiSel
Wickham H (2016) ggplot2: Elegant graphics for data analysis. Springer-Verlag, New York
Wiener P, Wilkinson S (2011) Deciphering the genetic basis of animal domestication. Proceedings of the Royal Society B: Biological Sciences, 278(1722), 3161–3170. doi:https://doi.org/10.1098/rspb.2011.1376
Wilbourn RV, Froy H, McManus M-C, Cheynel L, Gaillard J-M, Gilot-Fromont E, Regis C, Rey B, Pellerin M, Lemaître J-F, Nussey DH (2017) Age-dependent associations between telomere length and environmental conditions in roe deer. Biol Lett 13(9):20170434. doi:https://doi.org/10.1098/rsbl.2017.0434
Wilbourn RV, Moatt JP, Froy H, Walling CA, Nussey DH, Boonekamp JJ (2018) The relationship between telomere length and mortality risk in non-model vertebrate systems: a meta-analysis. Philos Trans R Soc Lond B Biol Sci 373(1741):20160447. doi:https://doi.org/10.1098/rstb.2016.0447
Wood EM, Capilla-Lasheras P, Cram DL, Walker LA, York JE, Lange A, Hamilton PB, Tyler CR, Young AJ (2021) Social dominance and rainfall predict telomere dynamics in a cooperative arid-zone bird. Mol Ecol. doi:https://doi.org/10.1111/mec.15868
Wright LI, Tregenza T, Hosken DJ (2007) Inbreeding, inbreeding depression and extinction. Conserv Genet 9(4):833. doi:https://doi.org/10.1007/s10592-007-9405-0
Wu X, Liu Y, Zhang Y, Gu R (2021) Advances in research on the mechanism of heterosis in plants. Front Plant Sci, 12, 74526. doi:https://doi.org/10.3389/fpls.2021.745726
Yang J, Lee SH, Goddard ME, Visscher PM (2011) GCTA: A tool for genome-wide complex trait analysis. Am J Hum Genet 88(1):76–82. doi:https://doi.org/10.1016/j.ajhg.2010.11.011
Young AJ (2018) The role of telomeres in the mechanisms and evolution of life-history trade-offs and ageing. Philos Trans R Soc Lond B Biol Sci 373(1741):20160452. doi:https://doi.org/10.1098/rstb.2016.0452
Acknowledgements
We thank everyone contributing to the fieldwork, Randi Røsbak, Anna S. Båtnes, and Linn-Karina Selvik for performing DNA extractions for genotyping procedures, and Pat Monaghan for facilitating telomere analyses at the University of Glasgow and for commenting on an early version of this manuscript.
Funding
This work was funded by the Research Council of Norway (274930 and 302619) and through its Centres of Excellence scheme (223257).
Open access funding provided by NTNU Norwegian University of Science and Technology (incl St. Olavs Hospital - Trondheim University Hospital)
Author information
Authors and Affiliations
Contributions
MLP measured telomeres, analyzed data, and wrote the manuscript with contributions from all authors. WB supervised telomere measurements. HJ, AKN, and TK contributed to the genotype data processing, pedigree construction, and in designing statistical analyses. THR, BE-S, and HJ initiated the study system. THR, HJ, and TK contributed to the fieldwork.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethics approval
Fieldwork was carried out in accordance with permits from the Ringing Centre at Stavanger Museum and the Norway Norwegian Animal Research Authority.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Thor Harald Ringsby and Henrik Jensen - Joint senior authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pepke, M.L., Niskanen, A.K., Kvalnes, T. et al. Inbreeding is associated with shorter early-life telomere length in a wild passerine. Conserv Genet 23, 639–651 (2022). https://doi.org/10.1007/s10592-022-01441-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-022-01441-x