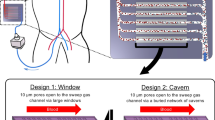
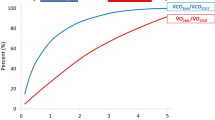
Abstract
While extracorporeal membrane oxygenation (ECMO) is a valuable therapy for patients with lung or heart failure, clinical use of ECMO remains limited due to hemocompatibility concerns with pro-coagulatory hollow fiber membrane geometries. Previously, we demonstrated the feasibility of silicon nanopore (SNM) and micropore (SμM) membranes for transport between two liquid-phase compartments in blood-contacting devices. Herein, we investigate various pore sizes of SNM and SμM membranes – alone or with a polydimethylsiloxane (PDMS) protective coating – for parameters that determine suitability for gas exchange. We characterized the bubble or rupture point of these membranes to determine sweep gas pressures at which gas emboli would form. The smallest pore size SNM and the SμM with PDMS coating could be pressurized in excess of 260 cmHg without rupture, which is comparable to hollow fiber sweep gas pressures. Oxygen flux for the SμM with and without PDMS was insignificantly different at 0.0306 ± 0.0028 and 0.0297 ± 0.0012 mL/min, respectively, while SNM flux was significantly lower at 0.0149 ± 0.0040 mL/min. However, the area-normalized mass transfer coefficient of the SNM was 338 ± 54 mL O2 m−2 min−1 cmHg−1 – an order of magnitude higher than that of the SμM with and without PDMS (57.3 ± 5.5 and 55.6 ± 2.2 mL O2 m−2 min−1 cmHg−1). Ultimately, we conclude that SμM-PDMS may make effective membranes for ECMO, since they are both mechanically robust and capable of high oxygen flux.







Similar content being viewed by others
References
J.V. Alexander, J.W. Neely, E.A. Grulke, J. Polym. Sci. Part B Polym. Phys. 52, 1366 (2014)
K.L. Brown, A.P. Goldman, Early Hum. Dev. 84, 143 (2008)
A. Dharia, E. Abada, B. Feinberg, T. Yeager, W. Moses, J. Park, C. Blaha, N. Wright, B. Padilla, S. Roy, Artif. Organs 42, 166 (2017)
W.H. Fissell, A. Dubnisheva, A.N. Eldridge, A.J. Fleischman, A.L. Zydney, S. Roy, J. Membr. Sci. 326, 58 (2009)
W.H. Fissell, A.T. Conlisk, S. Datta, J.M. Magistrelli, J.T. Glass, A.J. Fleischman, S. Roy, Microfluid. Nanofluid. 10, 425 (2011)
A.M. Gaffney, S.M. Wildhirt, M.J. Griffin, G.M. Annich, M.W. Radomski, Br. Med. J. 341, c5317 (2010)
D.M. Hoganson, J.L. Anderson, E.F. Weinberg, E.J. Swart, B.K. Orrick, J.T. Borenstein, J.P. Vacanti, J. Thorac. Cardiovasc. Surg. 140, 990 (2010)
D.M. Kanani, W.H. Fissell, S. Roy, A. Dubnisheva, A.L. Zydney, J. Memb. Sci. 349, 1 (2010)
S. Kim, B. Feinberg, R. Kant, B. Chui, K. Goldman, J. Park, W. Moses, C. Blaha, Z. Iqbal, C. Chow, N. Wright, W.H. Fissell, A. Zydney, S. Roy, PLoS One 11 (2016)
M.C. Kung, J.K. Lee, H.H. Kung, L.F. Mockros, ASAIO J 54 390 (2008)
S.W. Morley, P. Bieniek, M. Rosenberg, US 8,585,968 B2 (2013)
G.J. Peek, F. Clemens, D. Elbourne, R. Firmin, P. Hardy, C. Hibbert, H. Killer, M. Mugford, M. Thalanany, R. Tiruvoipati, A. Truesdale, A. Wilson, BMC Health Serv. Res. 13, 163 (2006)
J.A. Potkay, Biomed. Microdevices 15, 397 (2013)
J.A. Potkay, Lab Chip 14, 4122 (2014)
J.A. Potkay, M. Magnetta, A. Vinson, B. Cmolik, Lab Chip 11, 2901 (2011)
R.A. Smith, A.J. Fleischman, W.H. Fissell, C.A. Zorman, S. Roy, Meas. Sci. Technol. 22, 45802 (2011)
S. Song, C. Blaha, W. Moses, J. Park, N. Wright, J. Groszek, W. Fissell, S. Vartanian, A.M. Posselt, S. Roy, Lab Chip 17, 1778 (2017)
R.R. Thiagarajan, R.P. Barbaro, P.T. Rycus, D.M. McMullan, S.A. Conrad, J.D. Fortenberry, M.L. Paden, ASAIO J. 63, 60 (2017)
S. Vaquer, C. De Haro, P. Peruga, J.C. Oliva, A. Artigas, Ann. Intensive Care 7, 51 (2017)
T. Yeager, High Efficiency Asymmetric Membranes for Extracorporeal Membrane Oxygenation (University of California, San Francisco, 2017)
T. Yeager, S. Roy, Artif. Organs 41 (2017)
L. Zeman, J. Memb. Sci. 71, 233 (1992)
Acknowledgements
This research was supported by the National Heart, Lung, And Blood Institute of the NIH under Grant Number U54HL119893; NIH/NCATS UCSF-CTSI Grant Number UL1 TR001872; and US FDA Grant P50 FD003793 through the UCSF Pediatric Device Consortium. The membranes used in this study were designed and fabricated by JaeHyun Park of UCSF, and flow cells were designed by Nathan Wright of UCSF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abada, E.N., Feinberg, B.J. & Roy, S. Evaluation of silicon membranes for extracorporeal membrane oxygenation (ECMO). Biomed Microdevices 20, 86 (2018). https://doi.org/10.1007/s10544-018-0335-z
Published:
DOI: https://doi.org/10.1007/s10544-018-0335-z