Abstract
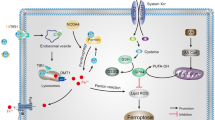
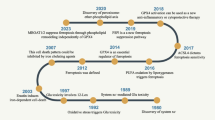
Ferroptosis, a mode of cell death that was recently identified in 2012, is driven by iron-dependent lipid peroxidation and distinct from other mechanisms of cell death such as autophagy and apoptosis. Ferroptosis has the unique features of disruptions in iron equilibrium, iron-induced lipid peroxidation, and the accumulation of glutamate-induced cellular toxicity. The regulation of ferroptosis mainly involves the iron, lipid, and amino acid metabolic pathways, which are controlled by system Xc−, voltage-dependent anion channels, p53 and other pathways. Neurodegenerative diseases involve gradual neuronal loss predominantly within the central nervous system and are categorized into both sporadic and rare hereditary disorders. These diseases result in the progressive decline of specific neuron populations and their interconnections. Recent investigations have revealed a strong correlation between the manifestation and progression of neurodegenerative diseases and ferroptosis. The pharmacological modulation of ferroptosis, whether by induction or inhibition, exhibits promising prospects for therapeutic interventions for these diseases. This review aims to examine the literature on ferroptosis and its implications in various neurodegenerative diseases. We hope to offer novel insights into the potential therapies targeting ferroptosis in central nervous system neurodegenerative diseases. However, there are still limitations of this review. First, despite our efforts to maintain objectivity during our analysis, this review does not cover all the studies on ferroptosis and neurodegenerative diseases. Second, cell death in neurodegenerative diseases is not solely caused by ferroptosis. Future research should focus on the interplay of different cell death mechanisms to better elucidate the specific disease pathogenesis.




Similar content being viewed by others
References
Xu J, Ma C, Hua M, Li J, Xiang Z, Wu J (2022) CNS and CNS diseases in relation to their immune system. Front Immunol 13:1063928
Li M, Wang ZW, Fang LJ, Cheng SQ, Wang X, Liu NF (2022) Programmed cell death in atherosclerosis and vascular calcification. Cell Death Dis 13(5):467
Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P et al (2018) Molecular mechanisms of cell death: recommendations of the nomenclature Committee on Cell Death 2018. Cell Death Differ 25:486–541
Hirschhorn T, Stockwell BR (2019) The development of the concept of ferroptosis. Free Radic Biol Med 133:130–143
Dolma S, Lessnick SL, Hahn WC, Stockwell BR (2003) Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3(3):285–296
Dixon SJ et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072
Distéfano AM, Martin MV, Córdoba JP, Bellido AM, D’Ippólito S, Colman SL, Soto D, Roldán JA, Bartoli CG, Zabaleta EJ, Fiol DF, Stockwell BR, Dixon SJ, Pagnussat GC (2017) Heat stress induces ferroptosis-like cell death in plants. J Cell Biol 216(2):463–476
Bogacz M, Krauth-Siegel RL (2018) Tryparedoxin peroxidase-deficiency commits trypanosomes to ferroptosis-type cell death. Elife 7:e37503
Shen Q, Liang M, Yang F, Deng YZ, Naqvi NI (2020) Ferroptosis contributes to developmental cell death in rice blast. New Phytol 227(6):1831–1846
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26(4):239–257
Qu M, Zhang H, Chen Z, Sun X, Zhu S, Nan K, Chen W, Miao C (2021) The role of ferroptosis in Acute Respiratory Distress Syndrome. Front Med (Lausanne) 8:651552
Ou M, Jiang Y, Ji Y, Zhou Q, Du Z, Zhu H, Zhou Z (2022) Role and mechanism of ferroptosis in neurological diseases. Mol Metab 61:101502
Kopeina GS, Zhivotovsky B (2022) Programmed cell death: past, present and future. Biochem Biophys Res Commun 633:55–58
Obeng E Apoptosis (programmed cell death) and its signals - a review. Braz J Biol 2021 Oct-Dec ;81(4):1133–1143
Brown CW, Amante JJ, Mercurio AM (2018) Cell clustering mediated by the adhesion protein PVRL4 is necessary for α6β4 integrin-promoted ferroptosis resistance in matrix-detached cells. J Biol Chem 293(33):12741–12748
Grzelak A, Wojewódzka M, Meczynska-Wielgosz S, Zuberek M, Wojciechowska D, Kruszewski M (2018) Crucial role of chelatable iron in silver nanoparticles induced DNA damage and cytotoxicity. Redox Biol 15:435–440
Fillebeen C, Charlebois E, Wagner J, Katsarou A, Mui J, Vali H, Garcia-Santos D, Ponka P, Presley J, Pantopoulos K (2019) Transferrin receptor 1 controls systemic iron homeostasis by fine-tuning hepcidin expression to hepatocellular iron load. Blood 133(4):344–355
Ohgami RS, Campagna DR, Greer EL, Antiochos B, McDonald A, Chen J, Sharp JJ, Fujiwara Y, Barker JE, Fleming MD (2005) Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet 37(11):1264–1269
Wang YQ, Chang SY, Wu Q, Gou YJ, Jia L, Cui YM, Yu P, Shi ZH, Wu WS, Gao G, Chang YZ (2016) The protective role of mitochondrial ferritin on Erastin-Induced ferroptosis. Front Aging Neurosci 8:308
Lou Z, Wang AP, Duan XM, Hu GH, Song GL, Zuo ML, Yang ZB (2018) Upregulation of NOX2 and NOX4 mediated by TGF-β signaling pathway exacerbates cerebral Ischemia/Reperfusion oxidative stress Injury. Cell Physiol Biochem 46(5):2103–2113
Li N, Wang W, Zhou H, Wu Q, Duan M, Liu C, Wu H, Deng W, Shen D, Tang Q (2020) Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic Biol Med 160:303–318
Masaldan S, Clatworthy SAS, Gamell C, Meggyesy PM, Rigopoulos AT, Haupt S, Haupt Y, Denoyer D, Adlard PA, Bush AI, Cater MA (2018) Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biol 14:100–115
Fuhrmann DC, Mondorf A, Beifuß J, Jung M, Brüne B (2020) Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol 36:101670
Ma S, Henson ES, Chen Y, Gibson SB (2016) Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis 7(7):e2307
Geng N, Shi BJ, Li SL et al (2018) Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells. Eur Rev Med Pharmacol Sci 22(12):3826–3836
Chen X et al (2021) Ferroptosis: machinery and regulation. Autophagy 17(9):2054–2081
Maiorino M, Conrad M, Ursini F (2018) GPx4, lipid peroxidation, and cell death: discoveries, Rediscoveries, and Open Issues. Antioxid Redox Signal 29(1):61–74
Li J, Jia B, Cheng Y, Song Y, Li Q, Luo C (2022) Targeting Molecular Mediators of Ferroptosis and Oxidative Stress for Neurological Disorders. Oxid Med Cell Longev. ; 2022:3999083
Tripathi R, Gupta R, Sahu M, Srivastava D, Das A, Ambasta RK, Kumar P (2022) Free radical biology in neurological manifestations: mechanisms to therapeutics interventions. Environ Sci Pollut Res Int 29(41):62160–62207
Raj S, Jaiswal SK, DePamphilis ML (2022) Cell death and the p53 Enigma during mammalian Embryonic Development. Stem Cells 40(3):227–238
Aubrey BJ, Kelly GL, Janic A, Herold MJ, Strasser A (2018) How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ 25(1):104–113
Jiang L, Hickman JH, Wang SJ, Gu W (2015) Dynamic roles of p53-mediated metabolic activities in ROS-induced stress responses. Cell Cycle 14(18):2881–2885
Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W (2015) Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520(7545):57–62
Gao M, Monian P, Quadri N, Ramasamy R, Jiang X (2015) Glutaminolysis and transferrin regulate ferroptosis. Mol Cell 59(2):298–308
Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB, Jiang X (2019) Role of Mitochondria in Ferroptosis. Mol Cell 73(2):354–363e3
Stockwell BR (2022) Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell 185(14):2401–2421
Hemono M, Ubrig É, Azeredo K, Salinas-Giegé T, Drouard L, Duchêne AM (2020) Arabidopsis Voltage-Dependent Anion channels (VDACs): overlapping and specific functions in Mitochondria. Cells 9(4):1023
Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I, Peltier JM, Boniface JJ, Smith R, Lessnick SL, Sahasrabudhe S, Stockwell BR (2007) RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447(7146):864–868
Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I et al (2019) FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575(7784):693–698
Doll S, Conrad M (2017) Iron and ferroptosis: a still ill-defined liaison. IUBMB Life 69(6):423–434
Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi AA, Lei P (2021) Ferroptosis: mechanisms and links with diseases. Signal Transduct Target Ther 6(1):49
Jiang X, Stockwell BR, Conrad M (2021) Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 22(4):266–282
Wells C, Brennan SE, Keon M, Saksena NK (2019) Prionoid proteins in the pathogenesis of neurodegenerative Diseases. Front Mol Neurosci 12:271
Sun Y, Xia X, Basnet D, Zheng JC, Huang J, Liu J (2022) Mechanisms of ferroptosis and emerging links to the Pathology of neurodegenerative Diseases. Front Aging Neurosci 14:904152
Dang X et al (2022) Correlation of ferroptosis and other types of cell death in neurodegenerative Diseases. Neurosci Bull 38(8):938–952
Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR (1995) An English translation of Alzheimer’s 1907 paper, Uber eine eigenartige Erkankung der Hirnrinde. Clin Anat 8(6):429–431
Breijyeh Z, Karaman R (2020) Comprehensive Review on Alzheimer’s Disease: causes and treatment. Molecules 25(24):5789
Lee AK, Khaled H, Chofflet N, Takahashi H (2020) Synaptic organizers in Alzheimer’s disease: a classification based on amyloid-b sensitivity. Front Cell Neurosci 14:281
Selkoe DJ, American College of Physicians; American Physiological Society (2004) Alzheimer disease: mechanistic understanding predicts novel therapies. Ann Intern Med 140(8):627–638
Long HZ, Cheng Y, Zhou ZW, Luo HY, Wen DD, Gao LC (2022) The key roles of organelles and ferroptosis in Alzheimer’s disease. J Neurosci Res 100(6):1257–1280
Kashani A, Lepicard E, Poirel O, Videau C, David JP, Fallet-Bianco C, Simon A, Delacourte A, Giros B, Epelbaum J, Betancur C, El Mestikawy S (2008) Loss of VGLUT1 and VGLUT2 in the prefrontal cortex is correlated with cognitive decline in Alzheimer disease. Neurobiol Aging 29(11):1619–1630
Ma H, Dong Y, Chu Y, Guo Y, Li L (2022) The mechanisms of ferroptosis and its role in Alzheimer’s disease. Front Mol Biosci 9:965064
Yoo SE, Chen L, Na R, Liu Y, Rios C, Van Remmen H et al Gpx4 ablation in adult mice results in a lethal phenotype accompanied by neuronal loss in brain. Free Radic Biol Med 52, 1820–1827
Bao WD, Pang P, Zhou XT, Hu F, Xiong W, Chen K, Wang J, Wang F, Xie D, Hu YZ, Han ZT, Zhang HH, Wang WX, Nelson PT, Chen JG, Lu Y, Man HY, Liu D, Zhu LQ (2021) Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ 28(5):1548–1562
Cardoso BR, Hare DJ, Bush AI, Roberts BR (2017) Glutathione peroxidase 4: a new player in neurodegeneration? Mol Psychiatry 22(3):328–335
Ramakrishna K, Nalla LV, Naresh D, Venkateswarlu K, Viswanadh MK, Nalluri BN, Chakravarthy G, Duguluri S, Singh P, Rai SN, Kumar A, Singh V, Singh SK (2023) WNT-β catenin signaling as a potential therapeutic target for neurodegenerative Diseases: current status and future perspective. Diseases 11(3):89
Hambright WS, Fonseca RS, Chen L, Na R, Ran Q (2017) Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol 12:8–17
Zhang YH, Wang DW, Xu SF, Zhang S, Fan YG, Yang YY, Guo SQ, Wang S, Guo T, Wang ZY, Guo C (2018) α-Lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S tau transgenic mice. Redox Biol 14:535–548
Peng W, Zhu Z, Yang Y, Hou J, Lu J, Chen C et al (2021) N2L, a novel lipoic acid-niacin dimer, attenuates ferroptosis and decreases lipid peroxidation in HT22 cells. Brain Res Bull 174:250–259
Rai SN, Singh P (2020) Advancement in the modelling and therapeutics of Parkinson’s disease. J Chem Neuroanat 104:101752
Brunden KR, Trojanowski JQ, Lee VM (2009) Advances in tau-focused drug discovery for Alzheimer’s disease and related tauopathies. Nat Rev Drug Discov 8(10):783–793
Parkinson J (2002) An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci 14:223–236
Fearnley JM, Lees AJ (1991) Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114(Pt 5):2283–2301
Jankovic J, Tan EK (2020) Parkinson’s disease: etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry 91(8):795–808
Hayes MT (2019) Parkinson’s disease and parkinsonism. Am J Med 132(7):802–807
Depierreux F et al (2021) Parkinson’s disease multimodal imaging: F-DOPA PET, neuromelanin-sensitive and quantitative iron-sensitive MRI. NPJ Parkinson’s Dis 7:57
Biondetti E et al (2021) The spatiotemporal changes in dopamine, neuromelanin and iron characterizing Parkinson’s disease. Brain 144:3114–3125
Mahoney-Sánchez L, Bouchaoui H, Ayton S, Devos D, Duce JA, Devedjian JC (2021) Ferroptosis and its potential role in the physiopathology of Parkinson’s Disease. Prog Neurobiol 196:101890
Costa I, Barbosa DJ, Benfeito S, Silva V, Chavarria D, Borges F, Remião F, Silva R (2023) Molecular mechanisms of ferroptosis and their involvement in brain diseases. Pharmacol Ther 244:108373
Powers KM, Smith-Weller T, Franklin GM, Longstreth WT Jr, Swanson PD, Checkoway H (2009) Dietary fats, cholesterol and iron as risk factors for Parkinson’s disease. Parkinsonism Relat Disord 15(1):47–52
Belaidi AA, Bush AI (2016) Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: targets for therapeutics. J Neurochem 139(Suppl 1):179–197
Haacke EM, Cheng NY, House MJ, Liu Q, Neelavalli J, Ogg RJ, Khan A, Ayaz M, Kirsch W, Obenaus A (2005) Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging 23(1):1–25
Vallerga CL, Zhang F, Fowdar J, McRae AF, Qi T, Nabais MF, Zhang Q, Kassam I, Henders AK, Wallace L, Montgomery G, Chuang YH, Horvath S, Ritz B, Halliday G, Hickie I, Kwok JB, Pearson J, Pitcher T, Kennedy M, Bentley SR, Silburn PA, Yang J, Wray NR, Lewis SJG, Anderson T, Dalrymple-Alford J, Mellick GD, Visscher PM, Gratten J (2020) Analysis of DNA methylation associates the cystine-glutamate antiporter SLC7A11 with risk of Parkinson’s disease. Nat Commun 11(1):1238
Pearce RK, Owen A, Daniel S, Jenner P, Marsden CD (1997) Alterations in the distribution of glutathione in the substantia nigra in Parkinson’s disease. J Neural Transm (Vienna) 104(6–7):661–677
Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD (1994) Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol 36(3):348–355
Sofic E, Lange KW, Jellinger K, Riederer P (1992) Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson’s disease. Neurosci Lett 142(2):128–130
Dexter D, Carter C, Agid F, Agid Y, Lees AJ, Jenner P, Marsden CD (1986) Lipid peroxidation as cause of nigral cell death in Parkinson’s disease. Lancet 2(8507):639–640
Do Van B, Gouel F, Jonneaux A, TimMerman K, Gelé P, Pétrault M, Bastide M, Laloux C, Moreau C, Bordet R, Devos D, Devedjian JC (2016) Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol Dis 94:169–178
Prakash J, Chouhan S, Yadav SK, Westfall S, Rai SN, Singh SP (2014) Withania somnifera alleviates parkinsonian phenotypes by inhibiting apoptotic pathways in dopaminergic neurons. Neurochem Res 39(12):2527–2536
Rai SN, Yadav SK, Singh D, Singh SP (2016) Ursolic acid attenuates oxidative stress in nigrostriatal tissue and improves neurobehavioral activity in MPTP -induced parkinsonian mouse model. J Chem Neuroanat 71:41–49
Yadav SK, Rai SN, Singh SP (2017) Mucuna pruriens reduces inducible nitric oxide synthase expression in parkinsonian mice model. J Chem Neuroanat 80:1–10
Tian Y, Lu J, Hao XQ, Li H, Zhang GY, Liu XL et al (2020) FTH1 inhibits ferroptosis through ferritinophagy in the 6-OHDA model of Parkinson’s disease. Neurotherapeutics 17:1796e1812
Koehler PJ, Jennekens FG (2008) Vinken and Bruyn’s handbook of clinical neurology. A witness of late-twentieth century neurological progress. J Hist Neurosci 17(1):46–55
Huang L, Fang L, Liu Q, Torshizi AD, Wang K (2021) Integrated analysis on transcriptome and behaviors defines HTT repeat-dependent network modules in Huntington’s disease. Genes Dis 9(2):479–493
Telenius H, Kremer B, Goldberg YP, Theilmann J, Andrew SE, Zeisler J, Adam S, Greenberg C, Ives EJ, Clarke LA et al (1994) Somatic and gonadal mosaicism of the Huntington disease gene CAG repeat in brain and sperm. Nat Genet 6(4):409–414
Lee J, Kosaras B, Del Signore SJ, Cormier K, McKee A, Ratan RR, Kowall NW, Ryu H (2011) Modulation of lipid peroxidation and mitochondrial function improves neuropathology in Huntington’s disease mice. Acta Neuropathol 121(4):487–498
Chen J, Marks E, Lai B, Zhang Z, Duce JA, Lam LQ, Volitakis I, Bush AI, Hersch S, Fox JH (2013) Iron accumulates in Huntington’s disease neurons: protection by deferoxamine. PLoS ONE 8(10):e77023
Klepac N, Relja M, Klepac R, Hećimović S, Babić T, Trkulja V (2007) Oxidative stress parameters in plasma of Huntington’s disease patients, asymptomatic Huntington’s disease gene carriers and healthy subjects: a cross-sectional study. J Neurol 254(12):1676–1683
Chen CM, Wu YR, Cheng ML, Liu JL, Lee YM, Lee PW, Soong BW, Chiu DT (2007) Increased oxidative damage and mitochondrial abnormalities in the peripheral blood of Huntington’s disease patients. Biochem Biophys Res Commun 359(2):335–340
Agrawal S, Fox J, Thyagarajan B, Fox JH (2018) Brain mitochondrial iron accumulates in Huntington’s disease, mediates mitochondrial dysfunction, and can be removed pharmacologically. Free Radic Biol Med 120:317–329
Mi Y, Gao X, Xu H, Cui Y, Zhang Y, Gou X (2019) The emerging roles of ferroptosis in Huntington’s Disease. Neuromolecular Med 21(2):110–119
Andrich J, Saft C, Gerlach M, Schneider B, Arz A, Kuhn W, Müller T (2004) Coenzyme Q10 serum levels in Huntington’s disease. J Neural Transm Suppl. ;(68):111–116
Mao Z, Choo YS, Lesort M (2006) Cystamine and cysteamine prevent 3-NP-induced mitochondrial depolarization of Huntington’s disease knock-in striatal cells. Eur J Neurosci 23(7):1701–1710
Johnson WM, Wilson-Delfosse AL, Mieyal JJ (2012) Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients 4(10):1399–1440
Rosas HD, Chen YI, Doros G, Salat DH, Chen NK, Kwong KK et al (2012) Alterations in brain transition metals in huntington disease an evolving and intricate story. Arch Neurol 69:887–893
van Bergen JM, Hua J, Unschuld PG, Lim IA, Jones CK, Margolis RL, Ross CA, van Zijl PC, Li X (2016) Quantitative susceptibility mapping suggests altered Brain Iron in Premanifest Huntington Disease. AJNR Am J Neuroradiol 37(5):789–796
Bartzokis G, Lu PH, Tishler TA, Fong SM, Oluwadara B, Finn JP, Huang D, Bordelon Y, Mintz J, Perlman S (2007) Myelin breakdown and iron changes in Huntington’s disease: pathogenesis and treatment implications. Neurochem Res 32(10):1655–1664
Simmons DA, Casale M, Alcon B, Pham N, Narayan N, Lynch G (2007) Ferritin accumulation in dystrophic microglia is an early event in the development of Huntington’s disease. Glia 55(10):1074–1084
Charvin D, Vanhoutte P, Pagès C, Borrelli E, Caboche J (2005) Unraveling a role for dopamine in Huntington’s disease: the dual role of reactive oxygen species and D2 receptor stimulation. Proc Natl Acad Sci U S A 102(34):12218–12223
Berggren KL, Chen J, Fox J, Miller J, Dodds L, Dugas B, Vargas L, Lothian A, McAllum E, Volitakis I, Roberts B, Bush AI, Fox JH (2015) Neonatal iron supplementation potentiates oxidative stress, energetic dysfunction and neurodegeneration in the R6/2 mouse model of Huntington’s disease. Redox Biol 4:363–374
Brown RH, Al-Chalabi A (2017) Amyotrophic lateral sclerosis. N Engl J Med 377:162–172
Hemerková P, Vališ M (2021) Role of oxidative stress in the pathogenesis of amyotrophic lateral sclerosis: antioxidant metalloenzymes and therapeutic strategies. Biomolecules 11:437
Deschauer M, Gaul C, Behrmann C, Prokisch H, Zierz S, Haack TB (2012) C19orf12 mutations in neurodegeneration with brain iron accumulation mimicking juvenile amyotrophic lateral sclerosis. J Neurol 259:2434–2439
Bonnefont-Rousselot D, Lacomblez L, Jaudon MC, Lepage S, Salachas F, Bensimon G et al (2000) Blood oxidative stress in amyotrophic lateral sclerosis. J Neurol Sci 178:57–62
Jeong SY, Rathore KI, Schulz K, Ponka P, Arosio P, David S (2009) Dysregulation of iron homeostasis in the CNS contributes to disease progression in a mouse model of amyotrophic lateral sclerosis. J Neurosci 29:610–619
Peng J, Pan J, Mo J, Peng Y (2022) MPO/HOCl facilitates apoptosis and ferroptosis in the SOD1(G93A) motor neuron of amyotrophic lateral sclerosis. Oxid. Med. Cell Longev. 2022:8217663
Matsuo T, Adachi-Tominari K, Sano O, Kamei T, Nogami M, Ogi K, Okano H, Yano M (2021) Involvement of ferroptosis in human motor neuron cell death. Biochem Biophys Res Commun 566:24–29
Chen X, Yu C, Kang R, Kroemer G, Tang D (2021) Cellular degradation systems in ferroptosis. Cell Death Differ 28:1135–1148
Devos D, Cabantchik ZI, Moreau C, Danel V, Mahoney-Sanchez L, Bouchaoui H et al (2020) Conservative iron chelation for neurodegenerative diseases such as Parkinson’s disease and amyotrophic lateral sclerosis. J Neural Transm 127:189–203
Spasić S, Nikolić-Kokić A, Miletić S, Oreščanin-Dušić Z, Spasić MB, Blagojević D, Stević Z (2020) Edaravone May prevent ferroptosis in ALS. Curr Drug Targets 21(8):776–780
Al-Chalabi A, Chiò A, Merrill C, Oster G, Bornheimer R, Agnese W et al (2021) Clinical staging in amyotrophic lateral sclerosis: analysis of Edaravone Study 19. J Neurol Neurosurg Psychiatry 92:165–171
Tamarit J, Obis È, Ros J (2016) Oxidative stress and altered lipid metabolism in Friedreich ataxia. Free Radic Biol Med 100:138–146
Cotticelli MG, Xia S, Lin D, Lee T, Terrab L, Wipf P, Huryn DM, Wilson RB (2019) Ferroptosis as a Novel Therapeutic Target for Friedreich’s Ataxia. J Pharmacol Exp Ther 369(1):47–54
Wong A, Yang J, Cavadini P, Gellera C, Lonnerdal B, Taroni F, Cortopassi G (1999) The Friedreich’s ataxia mutation confers cellular sensitivity to oxidant stress which is rescued by chelators of iron and calcium and inhibitors of apoptosis. Hum Mol Genet 8(3):425–430
Du J, Zhou Y, Li Y, Xia J, Chen Y, Chen S, Wang X, Sun W, Wang T, Ren X, Wang X, An Y, Lu K, Hu W, Huang S, Li J, Tong X, Wang Y (2020) Identification of Frataxin as a regulator of ferroptosis. Redox Biol 32:101483
Song X, Long D (2020) Nrf2 and ferroptosis: a New Research Direction for neurodegenerative Diseases. Front Neurosci 14:267
Lambiase A, Aloe L, Centofanti M, Parisi V, Báo SN, Mantelli F, Colafrancesco V, Manni GL, Bucci MG, Bonini S, Levi-Montalcini R (2009) Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: implications for glaucoma. Proc Natl Acad Sci U S A 106(32):13469–13474
Burton MJ, Ramke J et al (2021) The Lancet Global Health Commission on Global Eye Health: vision beyond 2020. Lancet Glob Health 9(4):e489–e551
Huang S, Liu K, Su Y, Wang F, Feng T (2023) Research progress of ferroptosis in glaucoma and optic nerve damage. Mol Cell Biochem 478(4):721–727
Kuehn MH, Fingert JH, Kwon YH (2005) Retinal ganglion cell death in glaucoma: mechanisms and neuroprotective strategies. Ophthalmol Clin North Am 18(3):383–395
Yao F, Peng J, Zhang E, Ji D, Gao Z, Tang Y, Yao X, Xia X (2023) Pathologically high intraocular pressure disturbs normal iron homeostasis and leads to retinal ganglion cell ferroptosis in glaucoma. Cell Death Differ 30(1):69–81
Lukasiewicz PD (2005) Synaptic mechanisms that shape visual signaling at the inner retina. Prog Brain Res 147:205–218
Zanon-Moreno V, Ciancotti-Olivares L, Asencio J, Sanz P, Ortega-Azorin C, Pinazo-Duran MD, Corella D (2011) Association between a SLC23A2 gene variation, plasma vitamin C levels, and risk of glaucoma in a Mediterranean population. Mol Vis 17:2997–3004
Ramdas WD (2018) The relation between dietary intake and glaucoma: a systematic review. Acta Ophthalmol 96(6):550–556
Velez-Montoya R, Oliver SC, Olson JL, Fine SL, Mandava N, Quiroz-Mercado H (2013) Current knowledge and trends in age-related macular degeneration: today’s and future treatments. Retina 33(8):1487–1502
Salvi SM, Akhtar S, Currie Z (2006) Ageing changes in the eye. Postgrad Med J 82(971):581–587
Ma W, Zhao L, Fontainhas AM, Fariss RN, Wong WT (2009) Microglia in the mouse retina alter the structure and function of retinal pigmented epithelial cells: a potential cellular interaction relevant to AMD. PLoS ONE 4(11):e7945
Ma W, Zhao L, Wong WT (2012) Microglia in the outer retina and their relevance to pathogenesis of age-related macular degeneration. Adv Exp Med Biol 723:37–42
Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC (2016) Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res 51:1–40
Dunaief JL, Richa C, Franks EP, Schultze RL, Aleman TS, Schenck JF, ZimMerman EA, Brooks DG (2005) Macular degeneration in a patient with aceruloplasminemia, a disease associated with retinal iron overload. Ophthalmology 112(6):1062–1065
Totsuka K, Ueta T, Uchida T, Roggia MF, Nakagawa S, Vavvas DG, Honjo M, Aihara M (2019) Oxidative stress induces ferroptotic cell death in retinal pigment epithelial cells. Exp Eye Res 181:316–324
Song D, Dunaief JL (2013) Retinal iron homeostasis in health and disease. Front Aging Neurosci 5:24
Sun Y, Zheng Y, Wang C, Liu Y (2018) Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis 9(7):753
Yang M, So KF, Lam WC, Lo ACY (2021) Cell ferroptosis: new mechanism and New Hope for Retinitis Pigmentosa. Cells 10(8):2153
Fahim A (2018) Retinitis pigmentosa: recent advances and future directions in diagnosis and management. Curr Opin Pediatr 30(6):725–733
He X, Hahn P, Iacovelli J, Wong R, King C, Bhisitkul R, Massaro-Giordano M, Dunaief JL (2007) Iron homeostasis and toxicity in retinal degeneration. Prog Retin Eye Res 26(6):649–673
Shu W, Baumann BH, Song Y, Liu Y, Wu X, Dunaief JL (2020) Ferrous but not ferric iron sulfate kills photoreceptors and induces photoreceptor-dependent RPE autofluorescence. Redox Biol 34:101469
Deleon E, LeDerman M, Berenstein E, Meir T, Chevion M, Chowers I (2009) Alteration in iron metabolism during retinal degeneration in rd10 mouse. Invest Ophthalmol Vis Sci 50(3):1360–1365
Huang B, Liang JJ, Zhuang X, Chen SW, Ng TK, Chen H (2018) Intravitreal Injection of Hydrogen Peroxide induces Acute Retinal Degeneration, apoptosis, and oxidative stress in mice. Oxid Med Cell Longev 2018:5489476
Lu L, Oveson BC, Jo YJ, Lauer TW, Usui S, Komeima K, Xie B, Campochiaro PA (2009) Increased expression of glutathione peroxidase 4 strongly protects retina from oxidative damage. Antioxid Redox Signal 11(4):715–724
Ueta T, Inoue T, Furukawa T, Tamaki Y, Nakagawa Y, Imai H, Yanagi Y (2012) Glutathione peroxidase 4 is required for maturation of photoreceptor cells. J Biol Chem 287(10):7675–7682
Zheng DW, Lei Q, Zhu JY, Fan JX, Li CX, Li C, Xu Z, Cheng SX, Zhang XZ (2017) Switching apoptosis to ferroptosis: Metal-Organic Network for High-Efficiency Anticancer Therapy. Nano Lett 17(1):284–291
Acknowledgements
We thank Professor. XianJun Zhu from Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China for providing long-term support in scientific research and constructive suggestions in this review.
Funding
This work was supported by The National Natural Science Foundation of China (82071009), the Department of Science and Technology of Qinghai Province (2022-HZ-814), program from the Department of Science and Technology of Sichuan Province (2023YFS0038).
Author information
Authors and Affiliations
Contributions
L.Z. designed and supervised the study. Y.W. and HJ.L. performed literature searching and wrote the majority of the manuscript. QX.H., R.Z. and JR.C. provided constructive advice on the revision of the draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Li, H., He, Q. et al. Ferroptosis: underlying mechanisms and involvement in neurodegenerative diseases. Apoptosis 29, 3–21 (2024). https://doi.org/10.1007/s10495-023-01902-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-023-01902-9