Abstract
Background and purpose
Estimation of the contractility of the left ventricle during exercise is important in drawing up a protocol of cardiac rehabilitation. It has been demonstrated that color Doppler- and echo tracking-derived carotid arterial wave intensity is a sensitive index of global left ventricular (LV) contractility. We assessed the feasibility of measuring carotid arterial wave intensity and determining force−frequency (contractility−heart rate) relations (FFRs) during exercise totally noninvasively.
Methods
We measured carotid arterial wave intensity with a combined color Doppler and echo tracking system in 25 healthy young male volunteers (age 20.8 ± 1.2 years) at rest and during exercise. FFRs were constructed by plotting the maximum value of wave intensity (WD1) against heart rate (HR).
Results
We first confirmed that HR increased linearly with an increase in work load in each subject (r 2 = 0.95 ± 0.04). WD1 increased linearly with an increase in HR. The goodness-of-fit of the regression line of WD1 on HR in each subject was very high (r 2 = 0.48−0.94, p < 0.0001, respectively). The slope of the WD1-HR relation ranged 0.30−2.20 [m/s3 (beat/min)].
Conclusions
Global LV FFRs can be generated in healthy young volunteers with an entirely noninvasive combination of exercise and wave intensity. These data should show the potential usefulness of the FFR in the context of cardiac rehabilitation.
Similar content being viewed by others
Introduction
Evaluation of the changes in the contractile state of the left ventricle during exercise is an important, and as yet not easy, goal in the procedure for cardiac rehabilitation. The positive inotropic effect of increasing the frequency of contraction in isolated cardiac muscles has been known as the Bowditch effect or the staircase phenomenon. The positive inotropic effect of increased heart rate (HR) produced by pacing also has been demonstrated in healthy human subjects, which has been called the force−frequency relation (FFR) [1, 2]. In conventional methods of obtaining FFRs, the maximum rate of left ventricular (LV) pressure rise (peak dP/dt) measured with a catheter-tipped micromanometer was used as an index of cardiac contractility, and atrial pacing was used to change HR (peak dP/dt−HR relation). It has also been reported that the FFR is significantly affected in hearts with a variety of diseases [3–7].
During exercise, HR increases with an increase in workload; therefore, atrial pacing is not needed for changing HR. It has also been demonstrated that the peak dP/dt−HR relation is markedly enhanced (the slope is increased) during exercise compared with during pacing in normal hearts, but the enhancement is limited in diseased hearts [8]. The FFR obtained by exercise may have higher power to discriminate cardiac contractile states than that obtained by pacing. In spite of its potential for evaluating cardiac function during exercise, peak dP/dt−HR relation has not been widely used because of its invasive nature. We have reported that carotid arterial wave intensity (WI) noninvasively measured with a combined system of color Doppler and echo tracking is a sensitive index of global LV contractile function (Fig. 1) [9, 10].
Relation between W1 obtained by carotid arterial echography and max dP/dt obtained with a catheter-tipped micromanometer (From Ohte N et al. [9])
In this study, we assessed the usefulness of WI to measure LV contractility and FFRs during exercise in healthy subjects, and evaluated the feasibility of an entirely noninvasive method for demonstration of the global LV FFR.
Materials and methods
Definition of wave intensity in terms of diameter-change
Wave intensity (WI) is a hemodynamic index, which is defined as
where dP/dt and dU/dt are the derivatives of blood pressure (P) and velocity (U) with respect to time, respectively. The maximum value of WI during a cardiac cycle (W1) significantly correlates with peak dP/dt (or max dP/dt) (Fig. 1) [9].
In our method of obtaining carotid arterial WI, the carotid diameter-change waveform was used as a surrogate for carotid pressure waveform. Using the diameter-change waveform directly, we can also define another wave intensity (WD) as
where D is the diameter. We can obtain the relation of WD to WI as follows:
The definition of the stiffness parameter, β, is written as
where D is the diameter at pressure P, and D d is the diameter at the end-diastolic pressure P d. The above equation gives:
The differentials of both sides of the above equation give
Since the difference between D and D d is small (Fig. 2), D d can be replaced by D. Then, we obtain
which gives
where dD/dt and dP/dt are the time derivatives of D and P, respectively. Substituting Eq. 3 into Eq. 2 and using Eq. 1, we obtain
Figure 2 shows the WD waveform calculated from the diameter-change waveform and the velocity waveform.
According to Eq. 4, the maximum value of carotid arterial WD during a cardiac cycle (WD1) correlates with the maximum value of WI (W1). Therefore, WD1 correlates with peak dP/dt as W1 does (Fig. 1). WD is obtained by measuring U and D without measuring upper arm pressure (Fig. 3), which is easier to perform during exercise. The details of the method of measurements were described elsewhere [11].
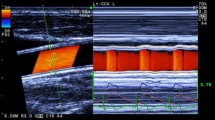
Measurements of diameter-change waveform and blood velocity. Left Long axis view of the common carotid artery and ultrasound beams. By setting the tracking positions displayed as small pink bars on the echo tracking beam (line A) to arterial walls, echo tracking automatically starts. The blood flow velocity averaged along the Doppler beam (line B) crossing the artery was measured using range-gated color Doppler signals. Right The diameter-change waveform, which is calculated by subtracting the distance to the near wall from that to the far wall, is displayed on the M-mode view. The blood flow velocity waveform is also displayed on the M-mode view
Thus, we can regard the WD1-HR relation as the FFR.
Subjects
We studied 25 healthy male volunteers (mean age 20.8 ± 1.2 years, age range 19−23 years) (Table 1). We obtained informed consent from all the subjects. Approval from the Ethics Committee of Himeji Dokkyo University was obtained before study initiation.
Protocol
Subjects were asked to refrain from caffeine, alcohol, and exercise during the 24-h period before the test. Before the measurements of WD1 and HR, the subjects were laid down the supine position for 10 min. The location to be measured was the right common carotid artery at about 2 cm proximal to the carotid bulb. We used scanning in the long axis view, and obtained a B-mode image of a longitudinal section of the artery (Fig. 3, left). With the B- and M-mode scans displayed simultaneously on a split screen, the echo tracking system tracked the vessel wall movements to produce displacement waveforms of the anterior and posterior artery walls (Fig. 3, right). This gave the diameter-change waveforms.
After the measurements of WD1 and HR at rest, gradual bicycle exercise was performed starting at an initial workload of 20 W and lasting for 2 min; thereafter, the workload was increased stepwise by 20 W at 1 min intervals. Electrocardiogram was continuously monitored. The criteria for the endpoint included increase of heart rate to [(220-age) × 0.8 (bpm)], and achievement of maximum fatigue or the impossibility of continuing exercise. We measured WD1 and HR during the exercise.
Statistical analysis
The obtained data are expressed as mean ± standard deviation. The scatter diagram of the points (WD1, HR) for the data during exercise from each subject was analyzed by the linear regression method, and the regression line was regarded as the FFR. A value of p < 0.05 was considered statistically significant.
Results
The baseline data from the subjects are shown in Table 1. We first confirmed that HR increased linearly with an increase in work load in all the subjects (r 2 = 0.95 ± 0.04) (Fig. 4). WD1 increased linearly with an increase in HR (Fig. 5). The goodness-of-fit of the regression line of WD1 on HR in each subject was very high (r 2 = 0.8 ± 0.1, p < 0.0001, respectively). The slope of the WD1-HR relation ranged from 0.30 to 2.20 [m/s3 (beat/min)] (mean ± SD: 1.0 ± 0.4 [m/s3 (beat/min)]). Figure 5 contains the WD1-HR relation with the greatest slope, that with the smallest slope, and those with moderate slopes.
Relations between the maximum values of WD (WD1) and heart rate (HR) (bpm). As representative data, WD1-HR relation with the greatest slope, that with the smallest slope, and those with moderate slopes are shown. The solid lines indicate the regression lines. The dotted curves show the 95 % confidence bands of the regression lines
Discussion
It has been reported that the basic property of the force−frequency effect to progressively enhance myocardial contractility as the heart rate increases is markedly augmented due to β-adrenergic stimulations induced by exercise in normal hearts [12, 13]. This possibility was examined in conscious dogs through the study of force−frequency effects during graded infusions of the β-adrenergic receptor agonist dobutamine under resting conditions. During infusions of low, medium, and high doses of dobutamine, the heart rate varied from 100 to 210 bpm by atrial pacing. During the dobutamine infusions, LV peak dP/dt progressively increased as the heart rate was augmented; the increase was greater at high doses than at low doses of dobutamine [14]. Thus, the regulatory effect of enhanced β-adrenergic stimulation on the FFR is as important as the basal FFR during exercise [15]. Significant impairment of β-adrenergic amplification of the FFR has been observed in heart failure, which could contribute to impaired cardiac contractility during exercise [16, 17]. Accordingly, one would expect that the diversity of responses of the FFR to β-adrenergic stimulations induced by exercise affords increased sensitivity for detection of contractile impairment.
One group has used exercise echocardiography to demonstrate FFRs noninvasively [18]. However, in contrast to the present study, their index (systolic blood pressure/end-systolic volume) was a rarely used and unvalidated index of contractility.
Several other groups have used tissue Doppler-derived isovolumic acceleration (IVA) during exercise, and presented FFR curves [19–21]. However, IVA is a parameter of regional myocardial contractility of the LV wall, and does not necessarily represent the global contractile state of the left ventricle. Indeed, IVA values vary considerably according to the region of interest of the LV wall. In some cases, IVA obtained from the mid lateral wall is more than 50 % greater than that obtained from the basal anterior wall [22]. In addition, the IVA-HR relation during exercise is not linear [21], in contrast to the linear WD1-HR relation. It is always easier to characterize a linear relation than to characterize a nonlinear relation.
Equation 4 shows that WD contains β. It is widely known that β increases with age. However, our study subjects only ranged from 19 to 23 years old. Therefore, β was considered to vary only slightly with the individual. In spite of this, the slope of the FFR varied relatively widely. This is considered to show that the FFR during exercise is determined mainly by the cardiac contractile state rather than arterial conditions.
Limitations
Although the WD1-HR relation depends on age, our study subjects only ranged from 19 to 23 years old. We should enroll a greater number of subjects to be divided into age groups in a future study. The final goal of our study is to apply the force−frequency relation to cardiac rehabilitation. However, we did not enroll patients with heart diseases in the present feasibility study.
Conclusions
Measurements of carotid arterial wave intensity by echocardiography during exercise give the FFR noninvasively. This will be useful for evaluation of the contractile state of the left ventricle during exercise for cardiac rehabilitation.
References
Anderson PA, Manring A, Johnson EA. Force-frequency relationship. A basis for a new index of cardiac contractility? Circ Res. 1973;33:665–71.
Anderson PA, Rankin JS, Arentzen CE, et al. Evaluation of the force-frequency relationship as a descriptor of the inotropic state of canine left ventricular myocardium. Circ Res. 1976;39:832–9.
Singal PK, Prasad K. Influence of ouabain on the force-frequency relationship in cardiac muscle. Jpn Heart J. 1979;20:341–50.
Szigligeti P, Pankucsi C, Banyasz T, et al. Action potential duration and force-frequency relationship in isolated rabbit, guinea pig and rat cardiac muscle. J Comp Physiol B. 1996;166:150–5.
Schmidt U, Schwinger RH, Bohm M. Halothane restores the altered force-frequency relationship in failing human myocardium. Anesthesiology. 1995;82:1456–62.
Monasky MM, Janssen PM. The positive force-frequency relationship is maintained in absence of sarcoplasmic reticulum function in rabbit, but not in rat myocardium. J Comp Physiol B. 2009;179:469–79.
Hasenfuss G, Holubarsch C, Hermann HP, et al. Influence of the force-frequency relationship on haemodynamics and left ventricular function in patients with non-failing hearts and in patients with dilated cardiomyopathy. Eur Heart J. 1994;15:164–70.
Miura T, Miyazaki S, Guth BD, et al. Influence of the force-frequency relation on left ventricular function during exercise in conscious dogs. Circulation. 1992;86:563–71.
Ohte N, Narita H, Sugawara M, et al. Clinical usefulness of carotid arterial wave intensity in assessing left ventricular systolic and early diastolic performance. Heart Vessels. 2003;18:107–11.
Sugawara M, Niki K, Ohte N, et al. Clinical usefulness of wave intensity analysis. Med Biol Eng Comput. 2009;47:197–206.
Niki K, Sugawara M, Chang D, et al. A new noninvasive measurement system for wave intensity: evaluation of carotid arterial wave intensity and reproducibility. Heart Vessels. 2002;17:12–21.
Mattera GG, Vanoli E, Martinez V, et al. Adrenergic effects on force-frequency relationship: a pivotal role for the cardiac intrinsic systems. Acta Physiol (Oxf). 2011;202:141–9.
Izawa H, Yokota M, Takeichi Y, et al. Adrenergic control of the force-frequency and relaxation-frequency relations in patients with hypertrophic cardiomyopathy. Circulation. 1997;96:2959–68.
Kambayashi M, Miura T, Oh B-H, et al. Enhancement of the force-frequency effect on myocardial contractility by adrenergic stimulation in conscious dogs. Circulation. 1992;86:572–80.
Ross JJr, Miura T, Kambayashi M, et al. Adrenergic control of the force-frequency relation. Circulation. 1995;92:2327–32.
Ross J Jr. Adrenergic regulation of the force-frequency effect. Basic Res Cardiol. 1998;93:95–101 (Epub 1998/12/02).
Matsuzaki M, Patritti J, Tajimi T, et al. Effects of beta-blockade on regional myocardial flow and function during exercise. Am J Physiol. 1984;247:H52–60.
Agricola E, Bombardini T, Oppizzi M, et al. Usefulness of latent left ventricular dysfunction assessed by Bowditch Treppe to predict stress-induced pulmonary hypertension in minimally symptomatic severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol. 2005;95:414–7.
Roche SL, Vogel M, Pitkanen O, et al. Isovolumic acceleration at rest and during exercise in children normal values for the left ventricle and first noninvasive demonstration of exercise-induced force-frequency relationships. J Am Coll Cardiol. 2011;57:1100–7.
Cheung MM, Smallhorn JF, Vogel M, et al. Disruption of the ventricular myocardial force-frequency relationship after cardiac surgery in children: noninvasive assessment by means of tissue Doppler imaging. J Thorac Cardiovasc Surg. 2006;131:625–31.
Vogel M, Cheung MM, Li J, et al. Noninvasive assessment of left ventricular force-frequency relationships using tissue Doppler-derived isovolumic acceleration: validation in an animal model. Circulation. 2003;107:1647–52.
Pauliks LB, Vogel M, Madler CF, et al. Regional response of myocardial acceleration during isovolumic contraction during dobutamine stress echocardiography: a color tissue Doppler study and comparison with angiocardiographic findings. Echocardiography. 2005;22:797–808.
Conflict of interest
We declare that we have no conflict of interest in connection with this paper.
Ethical considerations
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Tanaka, M., Sugawara, M., Ogasawara, Y. et al. Noninvasive evaluation of left ventricular force−frequency relationships by measuring carotid arterial wave intensity during exercise stress. J Med Ultrasonics 42, 65–70 (2015). https://doi.org/10.1007/s10396-014-0554-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-014-0554-8