Abstract
Background
The standard treatment for locally advanced esophageal cancer is preoperative chemotherapy with cisplatin and 5-fluorouracil (CF), followed by surgery. Although docetaxel plus cisplatin and 5-fluorouracil (DCF) has been reported to have favorable outcomes, no study has compared its therapeutic efficacy to that of standard treatment. This study aimed to compare the therapeutic effects of CF and DCF in the real world by matching patient background factors using propensity scores.
Methods
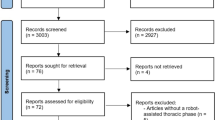
We retrospectively reviewed the data of 237 patients with esophageal squamous cell carcinoma who underwent esophagectomy between January 2008 and December 2018. Patients were divided into two groups based on the preoperative chemotherapy regimens of CF (79 patients) or DCF (158 patients), and 49 matched pairs were finally analyzed using propensity score matching. Short- and long-term outcomes were compared between groups.
Results
After matching, although no significant differences in survival were observed among the groups, patients receiving DCF showed a significantly high histological response (P < 0.001). Subgroup analyses demonstrated that DCF therapy had better overall survival (P = 0.046) and relapse-free survival (P = 0.010) among pathological T3 and T4 cases. Whereas, adverse effects of chemotherapy were more frequent in the DCF group.
Conclusions
Patients receiving DCF had higher pathological response and better survival than those receiving CF, especially in pathological T3 and T4 cases matched using propensity scores. Thus, the DCF regimen might be an effective treatment for locally advanced esophageal cancer. However, the adverse side effects of chemotherapy remain high and should be handled appropriately.




Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
Lagergren J, Smyth E, Cunningham D, et al. Oesophageal cancer. Lancet. 2017;390:2383–96. https://doi.org/10.1016/S0140-6736(17)31462-9.
Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68–74. https://doi.org/10.1245/s10434-011-2049-9.
Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: part 1. Esophagus. 2019;16:1–24. https://doi.org/10.1007/s10388-018-0641-9.
Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: part 2. Esophagus. 2019;16:25–43. https://doi.org/10.1007/s10388-018-0642-8.
Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan clinical oncology group study–JCOG9204. J Clin Oncol. 2003;21:4592–6. https://doi.org/10.1200/JCO.2003.12.095.
Yokota T, Hatooka S, Ura T, et al. Docetaxel plus 5-fluorouracil and cisplatin (DCF) induction chemotherapy for locally advanced borderline-resectable T4 esophageal cancer. Anticancer Res. 2011;31:3535–41.
Hashimoto M, Shirakawa Y, Maeda N, et al. Induction chemoradiotherapy including docetaxel, cisplatin, and 5-fluorouracil for locally advanced esophageal cancer. Esophagus. 2020;17:127–34. https://doi.org/10.1007/s10388-019-00709-5.
Yokota T, Kato K, Hamamoto Y, et al. Phase II study of chemoselection with docetaxel plus cisplatin and 5-fluorouracil induction chemotherapy and subsequent conversion surgery for locally advanced unresectable oesophageal cancer. Br J Cancer. 2016;115:1328–34. https://doi.org/10.1038/bjc.2016.350.
Yokota T, Kato K, Hamamoto Y, et al. A 3-year overall survival update from a phase 2 study of chemoselection with DCF and subsequent conversion surgery for locally advanced unresectable esophageal cancer. Ann Surg Oncol. 2020;27:460–7. https://doi.org/10.1245/s10434-019-07654-8.
Kato K, Ito Y, Daiko H, et al. A randomized controlled phase III trial comparing two chemotherapy regimen and chemoradiotherapy regimen as neoadjuvant treatment for locally advanced esophageal cancer JCOG1109 NExT study. J Clin Oncol. 2022;40:238. https://doi.org/10.1200/JCO.2022.40.4_suppl.238.
Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–61. https://doi.org/10.1002/pst.433.
Daiko H, Kato K. Updates in the 8th edtion of the TNM staging system for esophagus and esophagogastric junction cancer. Jpn J Clin Oncol. 2020;50:847–51.
Japan esophageal society. Japanese classification of esophageal cancer. 11th ed. Esophagus 2017;14:1–36.
Japan esophageal society. Japanese classification of esophageal cancer: II and III. 11th ed. Esophagus 2017;14:37–65.
Health U do SH. Common Terminol Criteria Adverse Events (CTCAE). 2017 Accessed 27 Nov 2017. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf;5:0
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8. https://doi.org/10.1038/bmt.2012.244.
Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 7th ed. Chichester, West Sussex, UK: Wiley-Blackwell; 2010.
Sobin LH, Wittekind C, editors. TNM classification of malignant tumors. 6th ed. New York: Wiley-Liss; 2002.
Lei N, Song Z, Lu B, et al. Docetaxel-based therapy with and without antiangiogenic agents as first-line chemotherapy for castration-resistant prostate cancer: a meta-analysis of nine randomized controlled trials. Mol Clin Oncol. 2014;2:1182–8. https://doi.org/10.3892/mco.2014.404.
Blom JW, Vanderschoot JP, Oostindiër MJ, et al. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost. 2006;4:529–35. https://doi.org/10.1111/j.1538-7836.2006.01804.x.
Haddad TC, Greeno EW. Chemotherapy-induced thrombosis. Thromb Res. 2006;118:555–68. https://doi.org/10.1016/j.thromres.2005.10.015.
Hamza MS, Mousa SA. Cancer-associated thrombosis: risk factors, molecular mechanisms, future management. Clin Appl Thromb Hemost. 2020;26:1076029620954282. https://doi.org/10.1177/1076029620954282.
Lechner D, Kollars M, Gleiss A, et al. Chemotherapy-induced thrombin generation via procoagulant endothelial microparticles is independent of tissue factor activity. J Thromb Haemost. 2007;5:2445–52. https://doi.org/10.1111/j.1538-7836.2007.02788.x.
Feffer SE, Carmosino LS, Fox RL. Acquired protein C deficiency in patients with breast cancer receiving cyclophosphamide, methotrexate, and 5-fluorouracil. Cancer. 1989;63:1303–7. https://doi.org/10.1002/1097-0142(19890401)63:7%3c1303::aid-cncr2820630713%3e3.0.co;2-f.
Guo W, Ma X, Yang S, et al. Combined thoracoscopic-laparoscopic esophagectomy versus open esophagectomy: a meta-analysis of outcomes. Surg Endosc. 2016;30:3873–81. https://doi.org/10.1007/s00464-015-4692-x.
Booka E, Haneda R, Ishii K, et al. The negative impact of preoperative chemotherapy on survival after esophagectomy for vulnerable elderly patients with esophageal cancer. Ann Surg Oncol. 2021;28:1786–95. https://doi.org/10.1245/s10434-020-09072-7.
Acknowledgements
The authors thank Prof. Hiroshi Morimatsu (Department of Anaesthesiology, Okayama University Graduate School of Medicine and Dentistry) for their expert cooperation during esophagectomy and perioperative care. We thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Contributions
Study conception and design: NN, KN, YS, and TF. Acquisition of data: NN, KN, TK, MH, NM, ST, and KS. Analysis and interpretation of data: NN, KN, YS, and TF. Drafting of manuscript: NN, KN, YS, and TF. Critical revision of manuscript: TK, MH, NM, ST, KS, YS, and TF.
Corresponding author
Ethics declarations
Ethical approval
This study was conducted in accordance with the principles of the Declaration of Helsinki. We obtained approval from the ethics committee of Okayama University Hospital (Approval No. 2109–011).
Conflict of interest
The authors declare no conflict of interest.
Informed consent
Due to the retrospective design of this study, informed consent from the patient was not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10388_2022_934_MOESM1_ESM.docx
Supplementary file1 Additional supporting information can be found in the Supporting Information section online (DOCX 5165 KB)
Rights and permissions
About this article
Cite this article
Nishiwaki, N., Noma, K., Kunitomo, T. et al. Neoadjuvant chemotherapy for locally advanced esophageal cancer comparing cisplatin and 5-fluorouracil versus docetaxel plus cisplatin and 5-fluorouracil: a propensity score matching analysis. Esophagus 19, 626–638 (2022). https://doi.org/10.1007/s10388-022-00934-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-022-00934-5