Abstract
Climate change is expected to intensify drought in the Mediterranean region. Previous studies indicate that tree species mixing may reduce the water stress. Our study investigates the response to past drought events of four co-occurring Mediterranean species: Pinus pinea L. (stone pine), Pinus pinaster Ait. (maritime pine), Juniperus thurifera L. (Spanish juniper) and Quercus ilex L (holm oak). The study was performed at an interannual scale, both in monospecific and mixed stands. Annual tree ring widths data measured on increment cores and stem discs obtained from 281 trees were used to quantify the responses to drought events using complementary resilience indices. Additionally, we assessed tree intra- and inter-specific competition impact over the past 25 years. We fitted and compared generalised linear mixed models to determine the influence of species identity, stand composition as intra-specific and inter-specific competition on complementary resilience indices and annual basal area increment. The co-existence with other species enhanced the resistance to drought of the stone pine as the resilience capacity of the Spanish juniper. Conversely, maritime pine’s drought resistance declined considerably in mixed stands. Notably, only the anisohydric species Spanish juniper and holm oak were able to return to pre-disturbance growth rates after the drought. The influence of competition on tree growth was found to differ according to the hydrological conditions of each year and varied based on the specific source of competition. Our study showed that mixed stands in the Spanish Northern Plateau, especially with holm oak and Spanish juniper, are more resilient to prolonged droughts due to spatio-temporal complementarity and subsequent competition reduction. That is another reason for which such composed mixed stands should be promoted in the arid conditions of the Spanish Northern Plateau.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forests on the Iberian Peninsula are increasingly subjected to more frequent and prolonged droughts (Senf et al. 2020). This climatic challenge, caused by decreased water supply combined with elevated atmospheric evaporative demand (Noto et al. 2023), is pushing even drought-adapted Mediterranean tree species towards their tolerance limits (Peñuelas et al. 2017). Mediterranean forests are experiencing a multifaceted impact due to increasing aridity, including shorter growth periods, accelerated soil erosion, nutrient imbalances and more frequent fires, all contributing to a significant dieback processes in tree species and altering forest dynamics (Peñuelas and Sardans 2021). The forests in the Spanish Northern Plateau are composed mainly by four species: Pinus pinea L. (stone pine), Pinus pinaster L. (maritime pine), Quercus ilex L. (holm oak) and Juniperus thurifera L. (Spanish juniper). Over the last 30 years, a progressive decline of Pinus pinaster has been observed in the region, both in resin-tapped stands (Calama et al. 2023) and mixed stands with Pinus pinea (Férriz et al. 2021). Furthermore, P. pinea, although more drought—resilient than P. pinaster, has shown signs of increased mortality (Férriz et al. 2021), a trend that may strengthen in the future due to climate change (Pardos et al. 2015).
Even among drought-resistant tree species present in the Spanish Northern Plateau (Lopez-Iglesias et al. 2014), substantial differences in drought responses are found. For instance, the isohydric stone pine and maritime pine will close their stomata sooner when air vapour pressure deficit will be increasing along with soil water potential decreasing; whereas the transpiration at high levels of hydraulic stress will be maintained by anisohydric holm oak and Spanish juniper (Pardos and Calama 2022). That gives an advantage to more drought-tolerant holm oak and Spanish juniper over both stone and maritime pine (Mayoral et al. 2016). This is one of the reasons behind a consequent replacement of several dominant Pinus species mainly by holm oak, across the lowlands of the Iberian Peninsula (Carnicer et al. 2014). Yet, it is important to point out that a single species does not represent entirely one or another strategy, but it moves in a continuum between one group of traits and another (Bredemeier et al. 2011).
As the performance of trees in mixtures often results in growth improvement (Condés et al. 2023), trees may deal better with water stress when growing with other species (Pardos et al. 2021a). The niche complementarity hypothesis offers an overarching explanation for this phenomenon. Resources may be taken up by trees at different points in space or time (resource partitioning) (Grossiord 2020), what contributes to competition reduction, when the inter-specific competition rate becomes lower than the intra-specific competition rate (Pretzsch et al. 2017; Ammer 2019). What is more, facilitation may occur—when the presence of one species leads to positive modification of growth environment of the remnant species. Competition reduction and facilitation are difficult to disentangle (Ammer 2019), but they can contribute to a better performance of mixed forest in overall (Pretzsch 2022), as under drought stress (Forrester et al. 2016). In Mediterranean mixed stands, Calama et al. (2021) and De Dios-García et al. (2018) demonstrated that stone pine growth was enhanced when coexisting with Spanish juniper and holm oak. Also, De Dios-García et al. (2015) confirmed better growth of stone pine when cohabiting with holm oak and Spanish juniper while facing water stress.
Secondary growth, an indicator of tree physiological response to drought stress, has been found to correlate positively with tree size and water supply in the Mediterranean region (Pasho et al. 2012; de-Dios-García et al. 2018; Martínez del Castillo et al. 2018; Calama et al. 2019). Complementary resilience indices based on ratios of secondary growth before, during, and after drought have been developed to quantify tree response to stress events (Lloret et al. 2011). Resistance shows tree’s ability to withstand the stress impact. Recovery indicates how effectively a tree is enhancing its performance after the damage suffered. Resilience demonstrates the capacity to reach pre-disturbance growth levels. Pardos et al. (2021a) showed that tree growth in mixed stands is less affected by drought stress, the effect being stronger in conifer-broadleaved mixtures compared to those composed by the same phylogenetic group of species (conifer-conifer or broadleaved-broadleaved). Tree hydraulic traits shape drought responses (Anderegg et al. 2016, 2018), thereby underscoring the importance of species’ identity in forming the mixture (Pardos et al. 2021a). The more different the hydraulic traits of trees are, the more pronounced the complementarity effect between species may become (Rodríguez de Prado et al. 2022; Haberstroh and Werner 2022). Species having its centre of distribution in the Mediterranean climate zone exhibit higher resilience (Pardos et al. 2021a) compared to species composing temperate forests, although this may decrease after repeated drought events (Serra-Maluquer et al. 2018). Broadleaved forests from temperate regions show greater resistance to drought, while conifer forests from arid and semi-arid regions are characterized by greater recovery. Nevertheless, if a species is existing at the limits of its distribution, even if that is an arid area, it may be more vulnerable to drought (Martínez del Castillo et al. 2022). Higher resistance to drought was proven to be linked to lower mortality likelihood in broadleaves, while conifer trees with higher recovery capacity are less likely to die due to drought in long term (DeSoto et al. 2020). Consecutive droughts have an accumulative effect on trees capacity to survive (Serra-Maluquer et al. 2018) and extend the recovery period (Haberstroh and Werner 2022), or can lead to a severe and critical growth decline (Schmied et al. 2023).
In the Mediterranean climate zone, trees mostly compete for water resources, with the intensity of competition being proportional (symmetrical) to their size (Bolibok 2014; de-Dios-García et al. 2015; Rodríguez de Prado et al. 2022). According to the stress gradient hypothesis, competition between trees is reduced with increasing stress rate (Forrester 2014). Competition may also shift to facilitation over time, such as during drought years (del Río et al. 2014). However, under extreme water stress, species interaction may return to be negative (Haberstroh and Werner 2022), as species better adapted to stress conditions will take advantage over those less adapted (del Castillo et al. 2016). Research indicates that higher competition rates limit trees’ annual growth during droughts (Dorman et al. 2015; Sohn et al. 2016), as in denser stands, quicker water depletion exacerbates this negative impact (Zhang et al. 2015).
However, the growth—competition dynamics is influenced by multiple factors, including species identity, stand structure and soil conditions (Mina et al. 2018; Vitali et al. 2018; Pretzsch 2022). In a meta-analysis study, Castagneri et al. (2022) found that denser stands showed lower resistance and, interestingly, resilience was not consistently enhanced by lower competition. On the contrary, increasing competition was observed to have a positive effect on recovery. Also, studies focusing on more specific cases report lower resilience and resistance rates with increasing competition (Bottero et al. 2017; Serra-Maluquer et al. 2018). Nevertheless, the majority of such studies focuses in pure or conifer-dominated stands. The interaction dynamics vary significantly across different forests composition. For instance, Rodríguez de Prado et al. (2022) observed in Spanish forests that conifers had higher basal area growth when mixed with broadleaved species, suggesting a shift from competition to neutralism or commensalism. In contrast, conifer-conifer stands predominantly exhibited competitive interactions. Furthermore, Condés et al. (2023) noted that in mixed stands with species of varying hydraulic traits, such as conifer or broadleaved species, the reduction in competition increased with aridity. However, comparative studies on the effects of competition on drought responses among Mediterranean tree species in mixed stands remain scarce.
Considering the increasing threat for Mediterranean forests, due to the shift in climate towards more aridity (Noto et al. 2023), our aim was to compare the response to drought and competition in terms of resilience indices and growth between mixed and pure stands of four co-occurring Mediterranean tree species. The species analysed were stone pine, maritime pine, Spanish juniper and holm oak, all widely present in the Spanish Northern Plateau, which is a region with Mediterranean continental climate. Our research is driven by the overarching question: How does the stand composition influence the annual growth and drought responses of these trees? We first explore this general inquiry, then delve deeper to unravel the nuances of competition, distinguishing between intra- and interspecific interactions. This approach aims to determine whether these competition sources impact differentially individual trees` growth rates and their responses to specific drought conditions. Reduced competition, or even a positive effect exerted by heterogenous species, especially during drought stress, could indirectly point to an existence of spatio-temporal complementarity mechanisms. Additionally, we aim to ascertain whether the impact of intra- and interspecific competition on annual basal area increment fluctuates over time. To attain the aforementioned objectives, we examined the following hypotheses:
Hypothesis I
Individual trees responses to drought events are conditioned by species identity and stand composition.
Hypothesis II
The annual basal area increment of individual trees is affected by the composition of the stand and species identity, along the entire study period, not only during drought events.
Hypothesis III
The effect of competition on a tree response to drought depends on the identity of the competitors (inter vs intraspecific competition).
Hypothesis IV
The competition affects the annual basal area increment of individual trees differently, depending on the identity of the competitors.
Hypothesis V
The influence of intra- and interspecific competition on the annual basal area increment of trees varies over different years.
Material and methods
Study site and climate
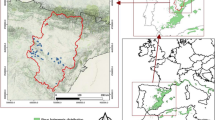
Data were collected in “El Carrascal” public forest (41°35′17.9″N 4°21′28.0″W), which is situated in the Northern Plateau of the Iberian Peninsula, occupying the watershed of the Duero River, in the province of Valladolid. The forested area of El Carrascal occupies 1140.19 ha, enclosed in a uniform woodland. The forest is composed of stone pine, maritime pine, Spanish juniper and holm oak. The study site is located on a flat area at an altitude of 886 m a.s.l. Soils are mostly arkosic sands and calcareous cambisols. El Carrascal was a private forest until 1985, and since then is owned and managed by the Regional Forest Service. While being a private forest, the management was focused on favouring timber and pine nut production in high stocking and even-aged stone pine stands. Meantime, the other species were either used for fuelwood or directly removed (de-Dios-García et al. 2015). Since 1985 the management efforts have gone towards promoting mixed stands, where maritime pine, holm oak and Spanish juniper grow together with stone pine. Some stands have not been managed in the last 25 years.
Climate is continental Mediterranean. During the period of study (1996–2021) the mean temperature was 12 °C and annual precipitation sum averaged 444.2 mm. The maximum absolute temperature averaged to 32 °C in June–August, while minimum absolute temperature to − 4 °C in December-February. The water deficit was occurring from February until October, the driest months being from June to September, when the monthly sum of precipitation varied between 18 and 27 mm per month and the climatic water balance (calculated as the difference between precipitation and potential evapotranspiration, following Penman—Monteith method (Allen et al. 1998)) fell beyond − 100 mm per month (Supplementary Material, Table S1). Climate data were obtained from “Valbuena del Duero” station (41°38′33″N 4°17′28″O; 737 m. a.s.l, www.inforiego.org), located at 5 km from the study site.
Experimental design
Seven rectangular plots with an area ranging from 0.15 to 0.48 ha (average 0.29 ha) were established in 2021 (Table 1). Four plots represent pure stands of the analysed species: stone pine, maritime pine, Spanish juniper and holm oak; three plots represent the most common available mixtures of the studied species within the site: one plot with stone pine—maritime pine—holm oak mixture and two plots with stone pine—Spanish juniper—holm oak composition with different degree of mixture. A stand was considered as pure if the dominant species represent at least 70% of the total number of stems. The plots were located in areas where no cuttings have been applied in the last 25 years, so the timespan for the analysis was restricted to 1996–2021. Every tree on a plot with diameter at breast height (DBH; cm) over 7 cm (Pinus spp.) or 5 cm (holm oak and Spanish juniper) was tagged and positioned, and DBH and total height (H; m) were measured (Table 1). Two DBH thresholds were used due to differences in the potential sizes reached by the different species. For the stone pine, maritime pine and Spanish juniper, two increment cores at breast height were extracted from 30 to 42 trees per species in each plot, resulting in 512 cores from 256 trees. For holm oak, wooden discs were cut from 25 trees located in the different plots. As nearly all trees on the plots were inventoried (n = 894), it was feasible to categorize them into DBH classes tailored for each species and plot. These classes were determined by segmenting the full range DBH measurements from the minimum to the maximum into intervals approximately equal to the standard deviation. Subsequently, a proportional number of trees for coring was selected from each class to ensure the sampling encompassed the full spectrum of diameters present.
Dendrochronological analysis
Tree ring widths (TRW; mm) were measured using LINTAB Measuring Table (Rinntech®) with a precision of 0.01 mm. TRW series for each tree were averaged. Statistics commonly used in dendrochronology were calculated to assess the reliability of constructed TRW series (Table S3) (Cook and Kairiukstis 1990; Speer 2010). We also calculated Synchronous Growth Changes (SGC) between all pairs of singular TRW series in a plot–species cluster. SGC expresses the percentage of simultaneous annual growth changes which had the same direction (i.e., increasing, decreasing or steady) in both compared TRW series. Therefore it is an enhanced measure of similarity between tree rings patterns comparing to previously used GLK (Gleichläufigkeit) (Visser 2021).
The tree ring width series characteristics obtained through cross dating and ring width measurements are shown in Table S3. The Expressed Population Signal (EPS) surpassed the widely accepted threshold of 0.85 (Speer 2010), indicating a high common variability among TRW series in response to environmental conditions. The average correlation between the TRW series and their respective master chronology ranged from 0.56 to 0.81, with Synchronous Growth Changes (SGC) consistently exceeding 0.60.
Once the averaged TRW for each year t and tree i from the plot j (TRWijt) was calculated, DBH of the cored trees was reconstructed as:
where \(DBH_{2021;ij}\) represents the breast height diameter of the tree at the year of inventory (2021). Then basal area (BA; cm2) and basal area increment (BAI; cm2 × year−1) of each tree i on year t were calculated.
One step detrending of BAI series was applied using splines (67% of wavelength, 50% frequency response), to remove age related growth fluctuations (Cook and Kairiukstis 1990), in order to obtain a series of indexed basal area increment (IBAI). All dendrochronological analysis were performed using dplR package (Bunn and Korpela 2018) within the R for Statistical Computing Environment (version 4.2.2).
Plot backdating
In order to reconstruct the past state of non-cored trees within the plot and characterize past competition status between trees, we used the following backdating sequence. First, we used BAI data of cored trees to fit the following linear regression:
This model was fitted separately for each plot and species, in 5-year timespans. Only the oak data was merged, as the sample size was low (Table S3). The fitted models were then used to predict the basal area increment of the non-cored trees in five-year periods (2021–2016, 2016–2011, 2011–2006, 2006–2001, 2001–1996). All models explained a high portion of variation in the data (R2 > 0.8). Basal area of a non-cored tree in a given year was obtained by subtracting the accumulated BAI from the basal area corresponding to 2021. Finally, tree basal area in a given year is transformed to tree DBH. We acknowledge that the described approach is based on an assumption that the trees of similar size were growing at similar pace, a fact about which we cannot be certain. Nevertheless, we believe, the method presented let us to reconstruct the past size of trees with enough accuracy to reflect the changing competition conditions.
Selection of drought years
In order to identify those years during which tree growth was mainly supressed due to extreme drought events, we used two indices (Steckel et al. 2020): (1) Standardized Precipitation—Evapotranspiration Index (SPEI) (Vicente-Serrano et al. 2010) for identifying years with significant water shortage (drought events) and (2) Cropper Index (Cropper 1979) for determining those years when deep growth reduction had taken place (negative pointer years). SPEI was computed for 1, 3, 6 and 12 accumulation periods (Sect. "Introduction", SI). A year was classified as drought event if at least one month during the growing season (March—October, defined as period with average monthly temperature ≥ 8 °C) exhibited a minimum of two SPEI variations (SPEI-1/-3/-6/-12) ≤ − 1 (Table S2) (Potop et al. 2014). With respect to Cropper Index, a year was identified as a negative pointer year if at least 60% of the cored trees showed TRW in that year lower than 0.75 standard deviations from the mean growth, computed in 5 year moving window (Jetschke et al. 2019). If a year was reported simultaneously as drought event and negative pointer year, it was considered as drought year.
Statistical analyses considered six drought years: 1999, 2002, 2005, 2009, 2012, and 2017 (Fig. S1). Regardless of the SPEI formulation used, the years 1999, 2005, 2009, 2012, and 2017 were consistently identified as drought events (Table S2). Additionally, due to low precipitation sum (284.8 mm) and harsh hydrological conditions (SPEI-1 and SPEI-12 < − 1 for May, August and September), 2002 was also considered a drought year. The cumulative precipitation during selected drought years averaged 241.3 mm per year for the hydrologic season (October previous year to September of the current year), compared to 439.1 mm for the entire analysed period.
Quantification of competition between trees
With the aim of capturing the competition between trees, we used distance dependent Hegyi Competition Index (HGCI) (Hegyi 1974). The index was proven to capture well the competition between trees from arid regions (Contreras et al. 2011), and it was suitable for the stands under study (Table S4). The index represents the sum of the competition exerted by the n competing trees over the ith subject tree, and was computed for each tree and year of the considered timespan (1996–2021):
where distik is the distance between the ith subject tree and kth neighbour tree; DBHk and DBHi are the diameters at breast height of the competitor and subject tree, respectively.
During the calculation of HGCI, we first identified the competitors of every subject tree, defined as those trees which Influence Area (IA; m2) overlapped with the IA of the subject tree (Tomé and Burkhart 1989). The IA of a given tree was defined as the area of a circle centred on that tree and a radius equal to 30 times the DBH of the tree (de-Dios-García et al. 2015). Correction for boundary effect of the plot was included following Martin et al. (1977).
The overall competition suffered by each subject tree i was splitted up into the competition experimented from neighbour trees of a different species (interspecific competition, CI.inter) and from neighbour trees which belong to the same species (intraspecific competition, CI.intra).
We calculated not only the present competition levels each tree faces but also the competition experienced in the past. To this aim, we utilized the reconstructed diameters of non-cored trees from previous years, as detailed in the Sect. “Plot backdating” (see also Sect. “Material and Methods”, SI). In this reconstruction task, we could not directly account for the mortality processes within the sample plots. Nevertheless, no signs of mortality, such as dead trees or stumps were evident in the defined DBH thresholds, reflecting the absence of management practices on these plots for the past 25 years. The sole exception was plot nr 3 (Table 1), where standing dead trees were found and included in the inventory. This inclusion indirectly represents the competition those trees were exerting in the past.
Response of trees to drought
The response of trees to drought was quantified using complementary resilience indices (Lloret et al. 2011), computed using IBAI series, for the selected drought years. The values of growth before (IBAIPreDr) and after the drought (IBAIPostDr) were calculated as the average of growth attained during a reference period before or after drought, respectively. Therefore, a sound choice of the reference period length is crucial (Schwarz et al. 2020). While establishing the reference period, we computed average growth recovery time (Thurm et al. 2016) in order to see, how many years each species across different compositions needed to recuperate its pre-drought growth. None of the species needed more than 2 years to recuperate its growth (Table S6). The distance between identified drought years was no more than 3 years in the majority of cases (Fig. S1). In order to avoid the overlapping between reference periods and drought years, the final choice of 2 years as reference period was made. Average values of the complementarity resilience indices calculated for differing reference periods (2–8 years) are shown in Table S7.
The resistance index (Rt) is the ratio between growth during (IBAIDr) and before the disturbance (IBAIPreDr):
Rt shows the ability of a single tree to maintain its growth during stress, with values closer to zero indicating lower resistance.
Recovery index (Rc) relates the average growth of a tree after disturbance (IBAIPostDr) to the growth during the disturbance:
Rc describes the ability of a tree to resume growth after a stress event, so a greater index indicates a faster process of recuperation of the tree.
Finally, the resilience index (Rs) relates the average growth of a tree before a stress event with the average growth after the event:
Rs shows the “returning to balance” of a tree after suffering a disturbance. Rs values > 1 show higher growth rates of a tree than before the disturbance; values < 1 demonstrate persistent growth rate decline, and Rs = 1 means that the tree returned to average growth rate present before disturbance. As Rs itself does not take into account the damage suffered by the tree during the disturbance (Lloret et al. 2011), the use of relative resilience (RRs) index is recommended:
Model fit, evaluation and comparison
The response variables (resilience indices and basal area increment (BAI)) showed high right—skewness (Figures S2 & S3). To appropriately model these variables we chose Generalised Linear Mixed Models (GLMM) with Gamma (for variables other than RRs due to its potential for negative values) and Tweedie (suitable for RRs) conditional distributions, both employing natural logarithmic (loge) link-function (Zuur et al. 2013; Manrique-Alba et al. 2022). To ascertain the superiority of GLMM over a conventional Linear Mixed Model (LMM) approach, we also evaluated Gaussian distributions with loge and identity link-functions. The GLMMs with Gamma and Tweedie error distributions consistently outperformed the Gaussian models in terms of AIC (accuracy constrained by parsimony), thus validating our choice of these distributions for our data (Burnham et al. 2002). Then, we optimized the random effects structure for each hypothesis model set based on the lowest AIC (Fieberg 2022), using Restricted Maximum Likelihood approximation (REML) (Zuur et al. 2009). In a subsequent step, the models involved in every hypothesis were compared in terms of AIC, Kullback–Leibler distance (ΔAIC) and weighted AIC (wAIC) (Burnham et al. 2011). Ecological interpretation of the best-selected model for each hypothesis is detailed in Table 2.
Post-hoc analyses of the hypotheses were performed using two differing techniques. For Hypotheses I and II, we calculated contrasts of estimated marginal means (EMM) for different levels of nominal variables (species, type of the stand) using the emmeans package (Lenth 2023). For the Hypotheses III and IV, adjusted predictions at representative values were calculated to evaluate the effects of focal predictors, using the ggeffects (Lüdecke 2018) and marginaleffects packages (Arel—Bundock 2023). All GLMMs were fitted using the glmmTMB package (Brooks et al. 2017). The statistical analyses were conducted using the R Language for Statistical Computing (R Core Team 2022).
Data analysis Modelling approach
In order to contrast each of the hypotheses stated, we compared different nested GLMMs. Our modelling approach was structured to incrementally address the complexity of our research questions. Initially, in Hypotheses I and II, our focus was on discerning the influence of species identity through stand composition on resilience indices and annual growth throughout the study period. This foundational step was crucial to establish a baseline understanding. Subsequently, in Hypotheses III and IV, we expanded our analysis to examine how different sources of competition experienced by individual trees influence their responses to specific drought events and their overall annual growth. Finally, in our last hypothesis, we explored how the competition effects on annual basal area increment fluctuate over time, offering insights into the dynamic nature of forest competition across years. By adopting this sequential modelling strategy, we addressed potential cofounding factors in a structured manner, ensured that each step of our analysis built logically upon the previous one. That allowed us to clearly isolate and understand the primary effects before introducing additional, more complex interactions in the subsequent models. These considerations are reflected in the following hypotheses:
Hypothesis I
Individual tree responses to drought events are conditioned by species identity and stand composition.
The main goal behind this hypothesis was to find if the composition of the stand affects significantly the reaction of trees to drought (computed for the drought years), expressed through resilience indices. In order to test this, three models were compared:
RI stands for each resilience index modelled separately (Rt, Rc, Rs, RRs). Species is a factor with four levels, which represents the analysed species (Jt, Pp, Pt, Qi) and Type is a factor with two levels, representing the two types of the stand under study (Mixed, Pure); DBH is the diameter at breast height (cm); µ is the intercept and βn represent the parameter coefficient for the nth fixed effect; bi, bj and bt represents the tree, plot and year random effects; \(\varepsilon_{ijt}\) stands for the residual error term. From now on, subscripts i, j, t represent tree, sample plot and year respectively.
Hypothesis II
The annual basal area increment of individual trees is affected by the composition of the stand and species identity.
To test this hypothesis the following set of models were fitted and compared, using data from the studied years (1996–2021):
All the other symbols as above.
Hypothesis III
The effect of competition on a tree response to drought depends on the identity of the competitors (inter vs intraspecific competition).
To test this hypothesis, we compared a model with only DBH fixed effect (Eq. 7), assuming no effect of competition on response to drought, with two alternative models, one assuming no specific identity on competition (CIijt, Eq. 8) and other assuming competition splitted into interspecific (CI.interijt) and intraspecific (CI.intraijt) competition rate (Eq. 9). The hypothesis was contrasted separately for each species and each resilience index, only within the identified drought years.
Hypothesis IV
The competition affects the annual basal area increment of individual trees differently, depending on the identity of the competitors.
To contrast this hypothesis, we fitted two different models for annual basal area increment, one assuming no specific identity on competition and other assuming competition separated into inter—specific (CI.interijt) and intra—specific (CI.intraijt). In these models tree size expressed by basal area (BA; cm2) and climatic water balance (CWB; mm) during hydrologic season (October(t-1) to September(t)) were also included as explanatory variables. They were standardized around their mean (Bolker 2022). The analysis was performed for all the years of the analysed period (1996–2021), separately for each species. Other symbols as above.
Hypothesis V
The influence of intra- and interspecific competition on the annual basal area increment of trees varies over different years.
In order to check this hypothesis, we fitted models with random annual slopes affecting both components of competition: CI.intra and CI.inter, separately for each species. Then we compared it to a model adjusted previously by Eq. 11. The varying sign and magnitude of random slopes coefficients in Eq. 12 is interpreted as varying competition conditions along years:
where b3t and b4t represents the random year slope of inter /intra—specific competition. All the rest of symbols as above.
After the model fitting and selection process, the most accurate model for every hypothesis was checked in terms of correct residual distribution and temporal autocorrelation (package DHARMa, (Hartig 2022)), as was multicollinearity between predictors (package performance, (Lüdecke et al. 2021)).
Results
Responses to drought across stand compositions and species identity
All resilience indices were better explained by a model that included both Type and Species × Type terms (Eq. 3, Table S8). The only exception was relative resilience (RRs), which was not influenced by the type of the stand. The stone pine showed 5% higher resistance (Rt) in mixed stands compared to pure stands (p-value = 0.002) (Table 3). As well, the recovery (Rc) from drought for the stone pine in mixed stands was lower by 17% (p-value = 0.067). Resilience (Rs) to drought of the stone pine did not significantly differ between pure and mixed stands. In contrast, maritime pine displayed lower resistance (− 12%, p-value < 0.001) and resilience (− 9%, p-value = 0.006) but higher recovery (+ 30%, p-value = 0.03) compared to pure stands (Table 3). The holm oak exhibited much lower recovery (− 60%, p-value = 0.015) from drought in mixed stands. The Spanish juniper showed significantly higher resilience (+ 11%, p-value = 0.003) in mixed stands. It should be noted that full resilience (≥ 1) was achieved only by the Spanish juniper and the holm oak in mixed stands and solely by the holm oak in pure stands.
Influence of stand composition on the annual growth of trees
Annual basal area increment was better explained by model including Type and Type × Species terms, indicating a variation between species based on the type of stand (Eq. 6, Table S8). Yearly BAI for all analysed species was significantly higher in mixed stands, except for the maritime pine, where it was importantly lower (− 9.30 cm2 year−1, p-value < 0.001). Spanish juniper’s growth was almost twice higher in mixed than in pure stands (+ 1.21 cm2 year−1, p-value < 0.001). The stone pine’s and holm oak’s growth were also faster in mixed stands, by 1.94 cm2 year−1 (p-value = 0.095) and 0.71 cm2 year−1 (p-value = 0.063), respectively. Among all species, the stone pine and the maritime pine showed the highest yearly growth, both in mixed and pure stands (Table 3).
Tree response to drought under the influence of the competitors’ identity
The inclusion of separate CI.intra and CI.inter terms (Eq. 9) led to improved explanations of resistance for the stone pine and the maritime pine, as well as resilience of the Spanish juniper and the stone pine. Also, relative resilience of the Spanish juniper and recovery of the maritime pine were better explained by separated competition effects. On the other hand, including an overall competition term without differentiating between inter-specific and intra-specific (Eq. 8) provided better explanations for recovery and relative resilience of the stone pine, resilience of the maritime pine and relative resilience of the holm oak (Fig. S4). Competition had no significant effect on resistance and recovery of the Spanish juniper, neither on relative resilience of the maritime pine, or on resistance, recovery and resilience of the holm oak, as indicated by the best-fitted models (Eq. 7, Tables S5 & S8).
For the resilience and relative resilience indices of Spanish juniper, and the resistance and resilience indices of the stone pine, the intra-specific competition (CI.intra) showed a negative effect, whereas inter-specific competition (CI.inter) showed a neutral one (as they were not different from zero) On the other hand, a less depressing effect of intra-specific competition (comparing to inter-specific competition) was observed only for the resistance of the maritime pine (Table 4, Figs. 1 and 2). On the contrary, competition from heterogenous tree species (CI.inter) strengthened the resistance to drought of the stone pine and the recovery of the maritime pine. CI.inter had also a positive effect on recovery for the latter species, stronger than CI.inter (Fig. 1). Overall competition had a negative influence on resilience of the maritime pine and relative resilience of the holm oak. Positive effect of overall competition could be confirmed for stone pine’s recovery, whereas for its relative resilience the described effect could not be confirmed as significant (Table 4).
Predictions of resistance and recovery (solid line) conditioned on intra—specific competition rate (CI.intra) and inter—specific competition rate (CI.inter), across the species for which at least one of those predictors resulted to be significant (Hypothesis III, Table 4). The remnant covariate (CI.intra/CI.inter and DBH) held at their observed mean values. Confidence intervals (95%) are represented by the dashed lines. Predictions made only accounting for fixed effects
Predictions of resilience and relative resilience (solid line) conditioned on intra—specific competition rate (CI.intra) and inter—specific competition rate (CI.inter), across the species for which at least one of those predictors resulted to be significant (Hypothesis III, Table 4). The remnant covariate (CI.intra/CI.inter and DBH) held at their observed mean values. Confidence intervals (95%) are represented by the dashed lines. Predictions made only accounting for fixed effects
The size of trees shaped the responses to drought remarkably for both pines and the holm oak. The unique responses to drought affected were resistance or recovery. Varying size of Spanish juniper trees had no effect on any of drought responses. Solely the stone pines were becoming more resistant to drought with their size increasing, whereas bigger maritime pines and holm oaks showed less resistance to drought. Increasing tree size caused worse recovery after drought in the case of stone pines, while for holm oaks, the contrary: the bigger were the trees, the faster were recovering after drought stress (Table 4).
Influence of the competitor’s identity on tree annual growth
The models predicting annual basal area increment (BAI) for the stone pine and the Spanish juniper showed improved adjustment when including terms for intra- and interspecific competition (Eq. 11). However, for maritime pine’s and holm oak’s BAI, the model with an overall competition term (Eq. 10) was the most parsimonious (Tables S6 & S8).
As expected, a positive dependency was found between tree size and annual growth, and all species showed enhanced growth with an increase in water supply throughout the year (Table S10, Fig. 3).
Predictions for BAI = annual basal area increment at single tree level (solid line) conditioned on: a basal area (BA); b climatic water balance (CWB) (Hypothesis IV, Eqs. 10, 11). Both predictors were standardized (means and standard deviations respective to each species on text). 95% confidence intervals enclosed between dashed lines. All other predictors held at their mean values. Competition indices (CI.intra/inter or HGCI) were left at their original (observed) scale. Predictions do not take into account random effects
Stronger competition rates, independently of their origin, led to reduced annual growth across all species (Table S10, Fig. S5). Inter-specific competition had marginally greater negative impact on the Spanish juniper’s BAI than intra-specific (decreases by 8% and 7%, respectively). The stone pine’s BAI was supressed slightly stronger by competition coming from conspecifics than from other species (reduction of growth by 22.2% and 22.3% accordingly). The overall competition rate significantly reduced the annual growth rate of both species, by 18% for the maritime pine and 8% for the holm oak (Table S10, Fig. S5).
Temporal variations of the effect of intra- and inter-specific competition on annual growth across species
For all analysed species, incorporating year random slopes on intra- and inter-specific competition varying across the study period significantly improved model fit compared to models that did not account for temporal variation in competition (Eq. 12, Table S9).
Both intra-specific competition (CI.intra) and inter-specific competition (CI.inter) had a negative influence on yearly basal area increment (BAI) for all species. On average (main effect), both competition sources similarly suppressed BAI, except for the maritime pine, where CI.inter had a stronger effect than CI.intra (CI.inter = − 0.36 vs. CI.intra = − 0.17). For the stone pine (CI.inter = − 0.26, CI.intra = − 0.27), the Spanish juniper (CI.inter = − 0.07, CI.intra = − 0.07), and the holm oak (CI.inter = − 0.10, CI.intra = − 0.10), the decreasing influence on BAI was similar, regardless of its source (Table S11).
The negative influence of competition on growth showed temporal shifts, conditioned by the competition source and hydrological conditions. Despite the temporal shifts, the final effect of competition always remained negative on BAI. During periods of below-average precipitation, CI.inter pressure decreased, while CI.intra pressure increased for the stone pine and the Spanish juniper. Conversely, maritime pine experienced a stronger negative effect from CI.inter during more intense drought conditions. CI.intra showed minor shifts during the analysed period. For the holm oak, the negative influence of CI.intra on growth remained constant, while CI.inter varied inconsistently (Fig. 4).
Random slopes coefficients adjusted for intra—specific competition (CI.intra) and inter—specific competition (CI.inter) experienced by the stone pine (Pp), the Spanish juniper (Jt), the maritime pine (Pt) and the holm oak (Qi). When the line falls below 0 (black dashed line), the competition becomes harsher (influences more negatively the BAI) and vice versa. Drought years on x—axis marked by orange colour; positive years marked as blue colour
Discussion
Our findings showed that tree responses to drought (except RRs) and annual basal area increment vary between mixed and pure stands, thus confirming Hypotheses I and II. However, the specific response to stand composition differed depending on the species (Pardos et al. 2021a). Stand type's influence on species' drought reactions and growth is complex, modulated by different competition types (Pretzsch 2022; Condés et al. 2023), nevertheless inconsistently across species. Thus, Hypotheses III and IV are supported partially. The effects of different competition sources varied annually, being influenced by the hydrological conditions. In this context, Hypothesis V is validated.
Mixture enhances growth and resistance of the stone pine
The stone pine trees in mixed stands showed greater drought resistance than in pure stands, influenced positively by inter-specific competition (Tables 4). Vergarechea et al. (2021) concurred with this finding when analysing stone pine—maritime pine mixtures. This observation aligns with Pardos et al. (2021a) or Haberstroh and Werner (2022) who found that mixed forests were more resistant to both mild and severe droughts. However, while stone pine displayed enhanced drought resistance in mixed stands, its recovery rate was slower (Table 3), a common trade-off observed in different species (Schwarz et al. 2020).
Over the entire study period (1996–2021), the stone pine exhibited a higher growth rate in mixed stands (Table 3). This is in agreement with Calama et al. (2021), who observed enhanced growth of stone pine in mixed forests, especially when combined with the Spanish juniper. Our model (Eq. 11) supports this, revealing that conspecific competitors exert a slightly more negative impact on the stone pine’s BAI than inter-specific trees (Table S10). These results confirm our hypotheses (H II & H IV), underscoring stone pine’s better performance in mixed stands. Competition in Mediterranean mixed stands primarily is symmetric (de-Dios-García et al. 2015, 2018) therefore revolves around below-ground resources (del Río et al. 2016). Hence, we believe that the presence of holm oak, which has a different water use strategy than stone pine (Mayoral et al. 2015) and uses the water pool from deeper soil layers (Vicente et al. 2018), may result in hydraulic lift and water release (Muñoz-Gálvez et al. 2021). Thus, stone pine’s favourable response to drought in mixed stands might be influenced by complementary spatial interactions with coexisting species.
Maritime pine’s performance is aggravated in mixed stands
The maritime pine in mixed stands exhibited a notably lower drought resistance (Table 3), a fact related to the more negative impact exerted by interspecific competition (Table 4). Therefore, we can also confirm the statements of H I and H III for the maritime pine, revealing its more negative performance in mixed stands during dry conditions. Competition was found to be the prevalent interaction between conifers in mixed Mediterranean forests (Rodríguez de Prado et al. 2022). Accordingly, among heterogenous species, the stone pine was the main competitor of the maritime pine (Table S5), supressing its BAI almost twofold stronger than conspecific neighbours (Vergarechea et al. 2021) (Table S11, Eq. 12), that effect being more pronounced in below-average water supply years (Fig. 4). Therefore, maritime pine’s BAI in mixed stands was reduced considerably (Table 3). Though the maritime pine is able to cope with individual drought events, it is highly vulnerable to repetitive drought stress (Madrigal-González and Zavala 2014) because of its conservative water use strategy (Ripullone et al. 2007), which might expose it to carbon starvation and eventual death (Salazar-Tortosa et al. 2018a). That explains increased maritime pine mortality when coexisting with stone pine (Férriz et al. 2021; Calama et al. 2023), and overall species decline in Central Spain (Gea-Izquierdo et al. 2021).
Spanish juniper is more resilient in mixtures, although its growth is inhibited under the overstorey of pines
The Spanish juniper showed non-significant differences between mixed and pure stands in terms of drought resistance (Table 3), a finding reinforced by the non-significant effect of competition on the resistance to drought (Table 4). More relevant observation is the enhanced resilience to drought observed in mixed stands compared to pure stands, supported by the highly negative impact of intraspecific competition over resilience (Table 4). Larger resilience of the Spanish juniper in mixed stands might stem from reduced resource competition following drought stress. This distribution could be due to the Spanish juniper tapping into the superficial ground stratum, while other species access deeper soil layers (de-Dios-García et al. 2015; Mayoral et al. 2016), being a clear sign of niche complementarity (Grossiord 2020). In addition, resilience of the Spanish juniper might be enhanced in the studied mature multi-layered stands, due to facilitation. As the Spanish juniper trees tend to occupy positions beneath stone pine’s crowns, the facilitation would consist in increased soil moisture due to canopy shading (Pardos et al. 2021b). What is more, increased survival rate of Juniperus spp. coexisting with Pinus spp. has been stated (Férriz et al. 2021), which may be coupled with the increased resilience to drought observed in our study (DeSoto et al. 2020).
With respect to growth, we detected a contradictory result: while in general BAI is almost twice larger in mixed stands, inter-specific competition exerts a greater suppressive effect, a phenomenon already described in Calama et al. (2021). A possible explanation for this contradiction may be due to the fact that pure Spanish juniper’ stands are only detected on very harsh and limiting microenvironments (low deep, rocky and less evolved soils), while when the soil and climate conditions are enhanced, the species tends to appear in mixture (Gauquelin et al. 1999). In mixed stands, the Spanish juniper, especially in young pole stages, tends to occupy clustered positions under stone pine’s crowns, where growth may be constrained due to severe competition (Pardos et al. 2021b).
Quercus ilex is largely indifferent to composition
The holm oak exhibited a heightened resistance to water stress when growing in mixed stands, yet the difference was not high enough to be considered as significant (Table 3). We could not observe a decreased impact of inter-specific competition on the resistance neither (Table 4). One possibility is that holm oak trees, being prominent resprouters (Rodà et al. 1999), grow in clusters. Consequently, the influence from neighbouring species on an individual holm oak tree might be muted, especially at smaller spatial scales. Supporting this, we observed a higher tree density in pure holm oak stands compared to mixed stands (Table 1). This higher density could intensify resource competition.
While annual basal area increment (BAI) of the holm oak was suppressed by competition in general, the identity of the neighbouring species did not seem to influence it (Table S10). This is evident from the near-identical impacts of inter and intra-species competition throughout our study (Table S11). Nevertheless, much higher holm oak’s BAI was observed in mixed than in pure stands (Table 3). The explanation seems to be quite simple: higher overall density in pure stands versus mixed stands aggravate the competition; which species is the competitor, has no meaning, as holm oak tends to form clusters, as mentioned before. Apart from the density reason, at some extent the holm oak may have more resources available while coexisting with other species. Two patterns, in line with the spatio-temporal niche complementarity theory (Ammer 2019; Grossiord 2020), might explain this phenomenon: first, stone pine, during water stress, limits its transpiration, reducing its water consumption. Second, while the Spanish juniper retains open stomata similar to holm oak, it predominantly relies on superficial water resources, which does not overlap with holm oak’s utilization of deeper soil moisture. Yet, our results cannot confirm the latter considerations.
Species comparison in performance facing drought: physiological implications
Of the four species we analysed, stone pine demonstrated significantly greater resistance to drought stress in mixed stands.
Maritime pine’s decreased resistance is understandable given stone pine’s suppressive effect on it (as previously discussed), making it the sole species with lower basal area increment in mixed stands. Vergarechea et al. (2021) found that in mixed stands, stone pine was less drought-resistant than maritime pine, but exhibited stronger post-drought recovery. Our findings echo this pattern, but only when the species are grown separately. When growing together, the two species display opposing drought responses: the stone pine showcases enhanced resistance (Perdiguero et al. 2015), but decreased recovery compared to the maritime pine. This divergence may be attributed to the stomatal behaviours of the two species. Stone pine may tend to close its stomata at more severe water stress levels than the maritime pine. The latter, having more strict stomatal control, potentially falls in an "isohydric trap"(Salazar-Tortosa et al. 2018b) leading to a prolonged period of photosynthetic inactivity, especially given the increasingly longer drought spells (Noto et al. 2023). That would explain the higher resistance rates in mixed stands, where the stone pine may be outperforming the maritime pine in terms of already scarce resource uptake (Salazar-Tortosa et al. 2018a). Both pines show similar rooting strategy (Andivia et al. 2019), therefore we can point out to differing transpiration strategies as the main differential factor between those species. The growth and eventual mortality of maritime pine were related to hydrological conditions of spring and winter (Prieto-Recio et al. 2015; Calama et al. 2023) while stone pine growth is mostly affected by total annual precipitation (Calama et al. 2021). Hence, we can conclude that stone pine is able to use autumn rainfalls more efficiently than maritime pine, which gives it another advantage during successively more dry climate conditions on the Iberian Peninsula.
Among the species analysed, Spanish juniper stands out with the highest drought resistance, irrespective of its growing environment. Its ability to maintain consistent photosynthesis rates, even under water stress (Pardos and Calama 2022) and its sensitiveness to any precipitation along the year (Férriz et al. 2021) lends it an advantage. Moreover, Spanish juniper sustain transpiration and subsequent photosynthesis and carbon allocation amidst severe water stress (Kukowski et al. 2013; Mayoral et al. 2016). This ability facilitates the consistent development of fine roots (Choat et al. 2018), albeit at slower rates compared to other species (Pardos and Calama 2022). Conversely, holm oak exhibited the lowest resistance but the highest recovery among the studied species. This is intriguing, given that holm oak was demonstrated to be even more drought tolerant than Juniperus spp., as it is capable of maintaining photosynthesis at much higher temperatures, also reaches deeper soil layers, thus preserving better water status (Mayoral et al. 2015). Then, we would expect from the holm oak resistance to drought similar or even higher than of the Spanish juniper.
A notable trend across our species is the trade-off between resistance and post-drought recovery. Generally, trees exhibiting reduced growth during disturbances (i.e., droughts) tend to recover faster from post-disturbance (Pretzsch et al. 2013). This inverse relationship between drought resistance and recovery is not only consistent across Northern Hemisphere forests (Gazol et al. 2017), but is also supported by meta-analyses focusing on these indices (Schwarz et al. 2020; Castagneri et al. 2022). Indeed, we observe higher recovery of the maritime pine growing in mixed stands along with the stone pine (Vergarechea et al. 2021). A complementary explanation would be the “return to competition” of maritime pine (Muñoz-Gálvez et al. 2021), resulting in fiercer contest for resources between maritime pine and stone pine after drought stress. That is, at some extent, mirrored in much stronger inter-specific competition impact on stone pine during improved water conditions (Fig. 4, Pp, i.e., positive years 2007–2008). Nevertheless, this pattern is not showed consequently by our results. Further investigation on the dependency between how competition rate is shaped by water conditions is needed. Notably, according to Gessler et al. (2020) slow recovery rate after suffering intense water stress may indicate trees are performing structural acclimation to future droughts.
In terms of resilience to drought events, the Spanish juniper and the holm oak were the standouts, achieving full resilience within the first two years after drought. In contrast, the stone pine and the maritime pine lagged, failing to match pre-drought growth rates in the given reference period (Table 3). This results are consistent with those reported by Piraino (2020) in stone pine stands for a 3-year reference period. Several factors may contribute to this difference in resilience between the studied species, although it must be subjected to further investigation. For instance, Vilalta et al. (2004) suggest that the wood of Pinus spp. is more prone to embolism, compared to the denser wood of drought-tolerant species like holm oak and Spanish juniper (Choat et al. 2005; Pacheco et al. 2016). Moreover, fine roots fraction of holm oak and Spanish juniper may be less impacted by prolonged drought (Choat et al. 2018), as these species do not cease carbon assimilation during water stress (Mayoral et al. 2016), unlike the pines. This difference could potentially affect the species post-drought competition for below-ground resources (Kukowski et al. 2013) and contribute to their varying levels of resilience.
Conclusions and final remarks
Our study conclusively indicates that in continental Mediterranean climate, mixture reinforces the resistance to drought of stone pine (de-Dios-García et al. 2015; Vergarechea et al. 2021). Extending previous studies on mature mixed stands in the region, we included holm oak and Spanish juniper in our analysis. These species demonstrated the highest levels of resilience to drought, with the Spanish juniper resilience further enhanced in mixed stands. Concurrently, our research supports predictions that more drought tolerant species, such as holm oak and Spanish juniper, may take advantage over species with stricter stomatal control, like stone pine (Pardos et al. 2021b). By incorporating competition source role in our study, we uncovered potential spatio- temporal complementarity processes that underpin the improved drought performance of the analysed species. These observations hold arising importance given the noted increasing drought durations and occurrences.
Clear silvicultural implications emerge from our research: advocating for and nurturing mixed stands, especially those accommodating understory species like holm oak and Spanish juniper, could bolster the stability and resilience of these stands. The decreased growth and resistance of maritime pine in mixed stands, as highlighted by Gea-Izquierdo et al. (2021) or Calama et al. (2023) and corroborated by our field observations, underscores the species’ gradual retreat from mixed stands shared with stone pine. It also confirms its vulnerability to intensifying climate conditions of Central Spain. Addressing potential shortcomings, our study would have benefited from a more detailed inter- specific competition index, divided into species-specific indexes. This division could provide a clearer understanding of the dynamic interplay between facilitation and competition among Mediterranean species. Integrating additional drought-resilience indices, such as those proposed by Thurm et al. (2016) and Schwarz et al. (2020), which expand on the widely adopted indices introduced by Lloret et al. (2011), might offer more comprehensive insights. Lastly, there is a palpable need for research that delves into the physiological processes of mature trees grown in mixed stands under water stress. Though challenging, such studies could illuminate the nuanced effects of facilitation between distinct species associations.
Data availability
References
Allen RG, Pereira LS, Smith M, Raes D (1998) Crop Evapotranspiration-Guidelines for computing crop water requirements-FAO Irrigation and Drainage Paper No. 56
Ammer C (2019) Diversity and forest productivity in a changing climate. New Phytol 221:50–66. https://doi.org/10.1111/nph.15263
Anderegg WRL, Klein T, Bartlett M et al (2016) Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc Natl Acad Sci USA 113:5024–5029. https://doi.org/10.1073/pnas.1525678113
Anderegg WRL, Konings AG, Trugman AT et al (2018) Hydraulic diversity of forests regulates ecosystem resilience during drought. Nature 561:538–541. https://doi.org/10.1038/s41586-018-0539-7
Andivia E, Zuccarini P, Grau B et al (2019) Rooting big and deep rapidly: the ecological roots of pine species distribution in southern Europe. Trees 33:293–303. https://doi.org/10.1007/s00468-018-1777-x
Arel - Bundock V (2023) Marginaleffects: predictions, comparisons, slopes, marginal means, and hypothesis tests
Bolibok L (2014) Przestrzenne uwarunkowania przemian składu gatunkowego drzewostanów Białowieskiego Parku Narodowego--: powstawanie, przeżywalność i awans dorostów = Spatial aspects of changes in species composition of natural forest stands in Białowieża National Park: ingrowths establishment, their survival, and advance to the canopy. Wydawnictwo SGGW, Warszawa
Bolker B (2022) GLMM FAQ. https://bbolker.github.io/mixedmodels-misc/glmmFAQ.html
Bottero A, D’Amato AW, Palik BJ et al (2017) Density-dependent vulnerability of forest ecosystems to drought. J Appl Ecol 54:1605–1614. https://doi.org/10.1111/1365-2664.12847
Bredemeier M, Cohen S, Godbold DL et al (eds) (2011) Forest management and the water cycle: an ecosystem-based approach. Springer, Netherlands
Brooks ME, Kristensen K, Benthem KJ et al (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378. https://doi.org/10.32614/RJ-2017-066
Bunn A, Korpela M (2018) Crossdating in dplR. 12
Burnham KP, Anderson DR, Burnham KP (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Burnham KP, Anderson DR, Huyvaert KP (2011) AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol 65:23–35. https://doi.org/10.1007/s00265-010-1029-6
Calama R, Conde M, de-Dios-García J et al (2019) Linking climate, annual growth and competition in a Mediterranean forest: Pinus pinea in the Spanish Northern Plateau. Agric for Meteorol 264:309–321. https://doi.org/10.1016/j.agrformet.2018.10.017
Calama R, de-Dios-García J, del Río M et al (2021) Mixture mitigates the effect of climate change on the provision of relevant ecosystem services in managed Pinus pinea L. forests. For Ecol Manag 481:118782. https://doi.org/10.1016/j.foreco.2020.118782
Calama R, Martínez C, Gordo J et al (2023) The impact of climate and management on recent mortality in Pinus pinaster resin-tapped forests of inland Spain. For Int J for Res. https://doi.org/10.1093/forestry/cpad023
Carnicer J, Coll M, Pons X et al (2014) Large-scale recruitment limitation in Mediterranean pines: the role of Quercus ilex and forest successional advance as key regional drivers. Glob Ecol Biogeogr 23:371–384. https://doi.org/10.1111/geb.12111
Castagneri D, Vacchiano G, Hacket-Pain A et al (2022) Meta-analysis reveals different competition effects on tree growth resistance and resilience to drought. Ecosystems 25:30–43. https://doi.org/10.1007/s10021-021-00638-4
Choat B, Ball MC, Luly JG, Holtum JAM (2005) Hydraulic architecture of deciduous and evergreen dry rainforest tree species from north-eastern Australia. Trees 19:305–311. https://doi.org/10.1007/s00468-004-0392-1
Choat B, Brodribb TJ, Brodersen CR et al (2018) Triggers of tree mortality under drought. Nature 558:531–539. https://doi.org/10.1038/s41586-018-0240-x
Condés S, Pretzsch H, del Río M (2023) Species admixture can increase potential tree growth and reduce competition. For Ecol Manag 539:120997. https://doi.org/10.1016/j.foreco.2023.120997
Contreras MA, Affleck D, Chung W (2011) Evaluating tree competition indices as predictors of basal area increment in western Montana forests. For Ecol Manag 262:1939–1949. https://doi.org/10.1016/j.foreco.2011.08.031
Cook ER, Kairiukstis LA (eds) (1990) Methods of dendrochronology. Springer, Netherlands
Cropper JP (1979) Tree-ring skeleton plotting by computer. Tree-Ring Bull 39:47–60
de-Dios-García J, Pardos M, Calama R (2015) Interannual variability in competitive effects in mixed and monospecific forests of Mediterranean stone pine. For Ecol Manag 358:230–239. https://doi.org/10.1016/j.foreco.2015.09.014
de-Dios-García J, Pardos M, Calama R et al (2018) A new multifactorial approach for studying intra-annual secondary growth dynamics in Mediterranean mixed forests: integrating biotic and abiotic interactions. Can J for Res 48:333–344. https://doi.org/10.1139/cjfr-2017-0139
del Castillo J, Comas C, Voltas J, Ferrio JP (2016) Dynamics of competition over water in a mixed oak-pine Mediterranean forest: Spatio-temporal and physiological components. For Ecol Manag 382:214–224. https://doi.org/10.1016/j.foreco.2016.10.025
del Río M, Schütze G, Pretzsch H (2014) Temporal variation of competition and facilitation in mixed species forests in Central Europe. Plant Biol 16:166–176. https://doi.org/10.1111/plb.12029
del Río M, Pretzsch H, Alberdi I et al (2016) Characterization of the structure, dynamics, and productivity of mixed-species stands: review and perspectives. Eur J Forest Res 135:23–49. https://doi.org/10.1007/s10342-015-0927-6
DeSoto L, Cailleret M, Sterck F et al (2020) Low growth resilience to drought is related to future mortality risk in trees. Nat Commun 11:545. https://doi.org/10.1038/s41467-020-14300-5
Dorman M, Perevolotsky A, Sarris D, Svoray T (2015) The effect of rainfall and competition intensity on forest response to drought: lessons learned from a dry extreme. Oecologia 177:1025–1038. https://doi.org/10.1007/s00442-015-3229-2
Férriz M, Martin-Benito D, Cañellas I, Gea-Izquierdo G (2021) Sensitivity to water stress drives differential decline and mortality dynamics of three co-occurring conifers with different drought tolerance. For Ecol Manage 486:118964. https://doi.org/10.1016/j.foreco.2021.118964
Fieberg J (2022) Statistics for ecologists: a frequentist and bayesian treatment of modern regression models: an open-source online textbook
Forrester DI, Bonal D, Dawud S et al (2016) Drought responses by individual tree species are not often correlated with tree species diversity in European forests. J Appl Ecol 53:1725–1734. https://doi.org/10.1111/1365-2664.12745
Forrester DI (2014) The spatial and temporal dynamics of species interactions in mixed-species forests: from pattern to process. For Ecol Manag 312:282–292. https://doi.org/10.1016/j.foreco.2013.10.003
Gauquelin T, Bertaudière-Montes V, Montes N et al (1999) Endangered stands of thuriferous juniper in the western Mediterranean basin: ecological status, conservation and management. Biodivers Conserv 8:1479–1498. https://doi.org/10.1023/A:1008966808796
Gazol A, Camarero JJ, Anderegg WRL, Vicente-Serrano SM (2017) Impacts of droughts on the growth resilience of Northern Hemisphere forests. Glob Ecol Biogeogr 26:166–176. https://doi.org/10.1111/geb.12526
Gea-Izquierdo G, Aranda I, Cañellas I et al (2021) Contrasting species decline but high sensitivity to increasing water stress on a mixed pine–oak ecotone. J Ecol 109:109–124. https://doi.org/10.1111/1365-2745.13450
Gessler A, Bottero A, Marshall J, Arend M (2020) The way back: recovery of trees from drought and its implication for acclimation. New Phytol 228:1704–1709. https://doi.org/10.1111/nph.16703
Grossiord C (2020) Having the right neighbors: how tree species diversity modulates drought impacts on forests. New Phytol 228:42–49. https://doi.org/10.1111/nph.15667
Haberstroh S, Werner C (2022) The role of species interactions for forest resilience to drought. Plant Biol J. https://doi.org/10.1111/plb.13415
Hartig F (2022) DHARMa: residual diagnostics for hierarchical (multi-level / mixed) regression models. In: DHARMa. http://florianhartig.github.io/DHARMa/. Accessed 13 Dec 2023
Hegyi F (1974) A simulation model for managing jackpine stands. In: Growth models for tree and stand simulation: proceedings of meetings in 1973. Royal College of Forestry
Jetschke G, van der Maaten E, van der Maaten-Theunissen M (2019) Towards the extremes: a critical analysis of pointer year detection methods. Dendrochronologia 53:55–62. https://doi.org/10.1016/j.dendro.2018.11.004
Kukowski KR, Schwinning S, Schwartz BF (2013) Hydraulic responses to extreme drought conditions in three co-dominant tree species in shallow soil over bedrock. Oecologia 171:819–830. https://doi.org/10.1007/s00442-012-2466-x
Lenth RV (2023) emmeans: estimated marginal means, aka least-squares means. R Package Version 1(8):6
Lloret F, Keeling EG, Sala A (2011) Components of tree resilience: effects of successive low-growth episodes in old ponderosa pine forests. Oikos 120:1909–1920
Lopez-Iglesias B, Villar R, Poorter L (2014) Functional traits predict drought performance and distribution of Mediterranean woody species. Acta Oecol 56:10–18. https://doi.org/10.1016/j.actao.2014.01.003
Lüdecke D (2018) ggeffects: tidy data frames of marginal effects from regression models. J Open Source Softw 3:772. https://doi.org/10.21105/joss.00772
Lüdecke D, Ben-Shachar MS, Patil I et al (2021) performance: an R package for assessment, comparison and testing of statistical models. J Open Source Softw 6:3139. https://doi.org/10.21105/joss.03139
Madrigal-González J, Zavala MA (2014) Competition and tree age modulated last century pine growth responses to high frequency of dry years in a water limited forest ecosystem. Agric for Meteorol 192–193:18–26. https://doi.org/10.1016/j.agrformet.2014.02.011
Manrique-Alba À, Beguería S, Camarero JJ (2022) Long-term effects of forest management on post-drought growth resilience: an analytical framework. Sci Total Environ 810:152374. https://doi.org/10.1016/j.scitotenv.2021.152374
Martin GR, Ek AR, Monserud RA (1977) Control of plot edge bias in forest stand growth simulation models. Can J for Res 7:100
Martínez del Castillo E, Tejedor E, Serrano-Notivoli R et al (2018) Contrasting patterns of tree growth of mediterranean pine species in the iberian Peninsula. Forests 9:416. https://doi.org/10.3390/f9070416
Martínez del Castillo E, Zang CS, Buras A et al (2022) Climate-change-driven growth decline of European beech forests. Commun Biol 5:1–9. https://doi.org/10.1038/s42003-022-03107-3
Martínez-Vilalta J, Sala A, Piñol J (2004) The hydraulic architecture of Pinaceae—a review. Plant Ecol 171:3–13. https://doi.org/10.1023/B:VEGE.0000029378.87169.b1
Mayoral C, Calama R, Sánchez-González M, Pardos M (2015) Modelling the influence of light, water and temperature on photosynthesis in young trees of mixed Mediterranean forests. New for 46:485–506. https://doi.org/10.1007/s11056-015-9471-y
Mayoral C, Pardos M, Sánchez-González M et al (2016) Ecological implications of different water use strategies in three coexisting mediterranean tree species. For Ecol Manag 382:76–87. https://doi.org/10.1016/j.foreco.2016.10.002
Mina M, Huber MO, Forrester DI et al (2018) Multiple factors modulate tree growth complementarity in Central European mixed forests. J Ecol 106:1106–1119. https://doi.org/10.1111/1365-2745.12846
Muñoz-Gálvez FJ, Herrero A, Esther Pérez-Corona M, Andivia E (2021) Are pine-oak mixed stands in Mediterranean mountains more resilient to drought than their monospecific counterparts? For Ecol Manag 484:118955. https://doi.org/10.1016/j.foreco.2021.118955
Nakagawa S, Johnson PCD, Schielzeth H (2017) The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J R Soc Interface 14:20170213. https://doi.org/10.1098/rsif.2017.0213
Noto LV, Cipolla G, Francipane A, Pumo D (2023) Climate change in the mediterranean basin (Part I): induced alterations on climate forcings and hydrological processes. Water Resour Manag 37:2287–2305. https://doi.org/10.1007/s11269-022-03400-0
Pacheco A, Camarero JJ, Carrer M (2016) Linking wood anatomy and xylogenesis allows pinpointing of climate and drought influences on growth of coexisting conifers in continental Mediterranean climate. Tree Physiol 36:502–512. https://doi.org/10.1093/treephys/tpv125
Pardos M, Calama R (2022) Adaptive strategies of seedlings of four mediterranean co-occurring tree species in response to light and moderate drought: a nursery approach. Forests 13:154. https://doi.org/10.3390/f13020154
Pardos M, Calama R, Maroschek M et al (2015) A model-based analysis of climate change vulnerability of Pinus pinea stands under multiobjective management in the Northern Plateau of Spain. Ann for Sci 72:1009–1021. https://doi.org/10.1007/s13595-015-0520-7
Pardos M, del Río M, Pretzsch H et al (2021a) The greater resilience of mixed forests to drought mainly depends on their composition: analysis along a climate gradient across Europe. For Ecol Manage 481:118687
Pardos M, Madrigal G, de Dios-García J et al (2021b) Sapling recruitment in mixed stands in the Northern Plateau of Spain: a patch model approach. Trees 35:2043–2058. https://doi.org/10.1007/s00468-021-02171-2
Pasho E, Camarero JJ, de Luis M, Vicente-Serrano SM (2012) Factors driving growth responses to drought in mediterranean forests. Eur J Forest Res 131:1797–1807. https://doi.org/10.1007/s10342-012-0633-6
Peñuelas J, Sardans J (2021) Global change and forest disturbances in the mediterranean basin: breakthroughs, knowledge gaps, and recommendations. Forests 12:603. https://doi.org/10.3390/f12050603
Peñuelas J, Sardans J, Filella I et al (2017) Impacts of global change on mediterranean forests and their services. Forests 8:463. https://doi.org/10.3390/f8120463
Perdiguero P, Soto Á, Collada C (2015) Comparative analysis of Pinus pinea and Pinus pinaster dehydrins under drought stress. Tree Genet Genomes 11:70. https://doi.org/10.1007/s11295-015-0899-1
Piraino S (2020) Assessing Pinus pinea L resilience to three consecutive droughts in central-western Italian Peninsula. For Biogeosci for 13:246. https://doi.org/10.3832/ifor3320-013
Potop V, Boroneanţ C, Možný M et al (2014) Observed spatiotemporal characteristics of drought on various time scales over the Czech Republic. Theor Appl Climatol 115:563–581. https://doi.org/10.1007/s00704-013-0908-y
Pretzsch H (2022) Facilitation and competition reduction in tree species mixtures in Central Europe: consequences for growth modeling and forest management. Ecol Model 464:109812. https://doi.org/10.1016/j.ecolmodel.2021.109812
Pretzsch H, Schütze G, Uhl E (2013) Resistance of European tree species to drought stress in mixed versus pure forests: evidence of stress release by inter-specific facilitation: drought stress release by inter-specific facilitation. Plant Biol 15:483–495
Pretzsch H, Forrester DI, Bauhus J (eds) (2017) Mixed-species forests. Springer, Berlin
Prieto-Recio C, Martín-García J, Bravo F, Diez JJ (2015) Unravelling the associations between climate, soil properties and forest management in Pinus pinaster decline in the Iberian Peninsula. For Ecol Manag 356:74–83. https://doi.org/10.1016/j.foreco.2015.07.033
R Core Team (2022) R: A language and environment for statistical computing
Ripullone F, Guerrieri MR, Nole A et al (2007) Stomatal conductance and leaf water potential responses to hydraulic conductance variation in Pinus pinaster seedlings. Trees 21:371–378. https://doi.org/10.1007/s00468-007-0130-6
Rodà F, Retana J, Gracia CA, Bellot J (eds) (1999) Ecology of mediterranean evergreen oak forests. Springer, Berlin
Rodríguez de Prado D, Riofrío J, Aldea J et al (2022) Competition and climate influence in the basal area increment models for Mediterranean mixed forests. For Ecol Manag 506:119955. https://doi.org/10.1016/j.foreco.2021.119955
Salazar-Tortosa D, Castro J, de Casas RR et al (2018a) Gas exchange at whole plant level shows that a less conservative water use is linked to a higher performance in three ecologically distinct pine species. Environ Res Lett 13:045004. https://doi.org/10.1088/1748-9326/aab18f
Salazar-Tortosa D, Castro J, Villar-Salvador P et al (2018b) The “isohydric trap”: A proposed feedback between water shortage, stomatal regulation, and nutrient acquisition drives differential growth and survival of European pines under climatic dryness. Glob Change Biol 24:4069–4083. https://doi.org/10.1111/gcb.14311
Schmied G, Pretzsch H, Ambs D et al (2023) Rapid beech decline under recurrent drought stress: individual neighborhood structure and soil properties matter. For Ecol Manag 545:121305. https://doi.org/10.1016/j.foreco.2023.121305
Schwarz J, Skiadaresis G, Kohler M et al (2020) Quantifying growth responses of trees to drought—a critique of commonly used resilience indices and recommendations for future studies. Curr for Rep 6:185–200. https://doi.org/10.1007/s40725-020-00119-2
Senf C, Buras A, Zang CS et al (2020) Excess forest mortality is consistently linked to drought across Europe. Nat Commun 11:6200. https://doi.org/10.1038/s41467-020-19924-1
Serra-Maluquer X, Mencuccini M, Martínez-Vilalta J (2018) Changes in tree resistance, recovery and resilience across three successive extreme droughts in the northeast Iberian Peninsula. Oecologia 187:343–354. https://doi.org/10.1007/s00442-018-4118-2
Sohn JA, Saha S, Bauhus J (2016) Potential of forest thinning to mitigate drought stress: a meta-analysis. For Ecol Manag 380:261–273. https://doi.org/10.1016/j.foreco.2016.07.046
Speer J (2010) Fundamentals of tree ring research. University of Arizona Press
Steckel M, del Río M, Heym M, et al (2020) Species mixing reduces drought susceptibility of Scots pine (Pinus sylvestris L.) and oak (Quercus robur L., Quercus petraea (Matt.) Liebl.) – Site water supply and fertility modify the mixing effect. For Ecol Manag 461:117908. https://doi.org/10.1016/j.foreco.2020.117908
Thurm EA, Pretzsch H, Uhl E (2016) Mixture reduces climate sensitivity of Douglas-fir stem growth. For Ecol Manag. https://doi.org/10.1016/j.foreco.2016.06.020
Tomé M, Burkhart HE (1989) Distance-Dependent Competition Measures for Predicting Growth of Individual Trees. Forest Sci 35:816–831. https://doi.org/10.1093/forestscience/35.3.816
Vergarechea M, Calama R, Pretzsch H et al (2021) Short- and long-term growth response to climate in mixed and monospecific forests of Pinus pinea and Pinus pinaster. Eur J Forest Res 140:387–402. https://doi.org/10.1007/s10342-020-01336-x
Vicente E, Vilagrosa A, Ruiz-Yanetti S et al (2018) Water Balance of Mediterranean Quercus ilex L. and Pinus halepensis Mill forests in semiarid climates: a review in a climate change context. Forests 9:426. https://doi.org/10.3390/f9070426
Vicente-Serrano SM, Beguería S, López-Moreno JI (2010) A multiscalar drought index sensitive to global warming: the standardized precipitation evapotranspiration index. J Clim 23:1696–1718. https://doi.org/10.1175/2009JCLI2909.1
Visser RM (2021) On the similarity of tree-ring patterns: assessing the influence of semi-synchronous growth changes on the Gleichläufigkeitskoeffizient for big tree-ring data sets. Archaeometry 63:204–215. https://doi.org/10.1111/arcm.12600
Vitali V, Forrester DI, Bauhus J (2018) Know your neighbours: drought response of norway spruce, silver fir and douglas fir in mixed forests depends on species identity and diversity of tree neighbourhoods. Ecosystems 21:1215–1229. https://doi.org/10.1007/s10021-017-0214-0
Zhang J, Huang S, He F (2015) Half-century evidence from western Canada shows forest dynamics are primarily driven by competition followed by climate. Proc Natl Acad Sci 112:4009–4014. https://doi.org/10.1073/pnas.1420844112
Zuur AF, Ieno EN, Walker N et al (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Zuur AF, Hilbe JM, Ieno EN (2013) A beginners guide to GLM and GLMM with R: a frequentist and bayesian perspective for ecologists. Highland Statistics Ltd, Newburgh
Acknowledgements
We thank the Forest Service of Junta de Castilla y León for facilitating the sample plots installation in El Carrascal Forest. We thank Sergio Bravo for help during plot measurements and Enrique Garriga and Monika Bradatsch for core preparation and reading.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. The study is supported by ETN Skill-For.Action project that has received funding from the European Union’s HORIZON 2020 research and innovation programme under the Marie Skłodowska Curie grant agreement No. 956355.
Author information
Authors and Affiliations
Contributions
Conceptualization MP and RC; methodology MP and RC; data collection—all authors; data curation PJ; formal analysis PJ; writing—original draft PJ; writing, review and editing PJ, MP and RC; project administration MP; supervision MP and RC. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The Authors declare no conflicts of interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Enno Uhl.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jankowski, P.A., Calama, R., Madrigal, G. et al. Enhanced interannual drought resilience in mixed stands: unveiling possible complementarity effects between tree species of the Spanish Northern Plateau. Eur J Forest Res (2024). https://doi.org/10.1007/s10342-024-01685-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10342-024-01685-x