Abstract
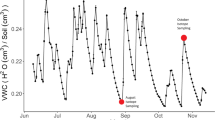
An important component of the hydrological niche involves the partitioning of water sources, but in landscapes characterized by shallow soils over fractured bedrock, root growth is highly constrained. We conducted a study to determine how physical constraints in the root zone affected the water use of three tree species that commonly coexist on the Edwards Plateau of central Texas; cedar elm (Ulmus crassifolia), live oak (Quercus fusiformis), and Ashe juniper (Juniperus ashei). The year of the study was unusually dry; minimum predawn water potentials measured in August were −8 MPa in juniper, less than −8 MPa in elm, and −5 MPa in oak. All year long, species used nearly identical water sources, based on stable isotope analysis of stem water. Sap flow velocities began to decline simultaneously in May, but the rate of decline was fastest for oak and slowest for juniper. Thus, species partitioned water by time when they could not partition water by source. Juniper lost 15–30 % of its stem hydraulic conductivity, while percent loss for oak was 70–75 %, and 90 % for elm. There was no tree mortality in the year of the study, but 2 years later, after an even more severe drought in 2011, we recorded 34, 14, 6, and 1 % mortality among oak, elm, juniper, and Texas persimmon (Diospyros texana), respectively. Among the study species, mortality rates ranked in the same order as the rate of sap flow decline in 2009. Among the angiosperms, mortality rates correlated with wood density, lending further support to the hypothesis that species with more cavitation-resistant xylem are more susceptible to catastrophic hydraulic failure under acute drought.






Similar content being viewed by others
References
Abbott PL (1975) Hydrology of Edwards limestone, south-central Texas. J Hydrol 24:251–269
Adams HD et al (2009) Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proc Natl Acad Sci USA 106:7063–7066
Allen CD et al (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manag 259:660–684
Araya YN, Silvertown J, Gowing DJ, McConway KJ, Linder HP, Midgley G (2011) A fundamental, eco-hydrological basis for niche segregation in plant communities. New Phytol 189:253–258
Barnes CJ, Walker GR (1989) The distribution of deuterium and O-18 during unsteady evaporation from a dry soil. J Hydrol 112:55–67
Bendevis MA, Owens MK, Heilman JL, McInnes KJ (2010) Carbon exchange and water loss from two evergreen trees in a semiarid woodland. Ecohydrology 3:107–115
Bleby TM, McElrone AJ, Jackson RB (2010) Water uptake and hydraulic redistribution across large woody root systems to 20 m depth. Plant Cell Environ 33:2132–2148
Chesson P et al (2004) Resource pulses, species interactions, and diversity maintenance in arid and semi-arid environments. Oecologia 141:236–253
Dietze MC, Moorcroft PR (2011) Tree mortality in the eastern and central United States: patterns and drivers. Glob Change Biol 17:3312–3326
Dixon R (2000) Climatology of the Freeman Ranch, Hays County, Texas. In: Freeman Ranch Publication Series, No. 3-2000. Southwest State University, San Marcos, Texas, pp 2–3
Donovan LA, Richards JH, Linton MJ (2003) Magnitude and mechanisms of disequilibrium between predawn plant and soil water potentials. Ecology 84:463–470
Duniway MC, Herrick JE, Monger HC (2007) The high water-holding capacity of petrocalcic horizons. Soil Sci Soc Am J 71:812–819
Ehleringer J, Roden J, Dawson T (2000) Assessing ecosystem level water relations through stable isotope ratio analysis. In: Sala O, Jackson R, Mooney H, Howarth R (eds) Methods in ecosystem science. Springer, New York, pp 181–214
Ellmore GS, Ewers FW (1985) Hydraulic conductivity in trunk xylem of elm, Ulmus americana. International Association of Wood Anatomists Bulletin 6:303–307
Ellmore GS, Ewers FW (1986) Fluid-flow in the outermost xylem increment of a ring-porous tree, Ulmus americana. Am J Bot 73:1771–1774
Evans RD, Ehleringer JR (1994) Water and nitrogen dynamics in an arid woodland. Oecologia 99:233–242
Fahlquist L, Ardis AF (2004) Quality of water in the Trinity and Edwards aquifers, South-Central Texas, 1996-98: U.S. Geological Survey Scientific Investigations Report 2004-5201, p 17
Fitter AH (1987) An architectural approach to the comparative ecology of plant-root systems. New Phytol 106:61–77
Fonteyn PJ, McClean TM, Akridge RE (1985) Xylem pressure potentials of 3 dominant trees of the Edwards Plateau of Texas. Southwest Nat 30:141–146
Gonfiantini R (1978) Standards for stable isotope measurements in natural compounds. Nature 271:534–536
Granier A (1985) A new method of sap flow measurement in tree stems. Ann Sci For 42:193–200
Hacke UG, Sperry JS (2001) Functional and ecological xylem anatomy. Perspect Plant Ecol Evol Syst 4:97–115
Hartmann H (2011) Will a 385 million year-struggle for light become a struggle for water and for carbon? How trees may cope with more frequent climate change-type drought events. Glob Change Biol 17:642–655
Hodge A, Berta G, Doussan C, Merchan F, Crespi M (2009) Plant root growth, architecture and function. Plant Soil 321:153–187
Hoffmann WA, Marchin RM, Abit P, Lau OL (2011) Hydraulic failure and tree dieback are associated with high wood density in a temperate forest under extreme drought. Glob Change Biol 17:2731–2742
Jackson RB, Moore LA, Hoffmann WA, Pockman WT, Linder CR (1999) Ecosystem rooting depth determined with caves and DNA. Proc Natl Acad Sci USA 96:11387–11392
Kattge J et al (2011) TRY—a global database of plant traits. Glob Change Biol 17:2905–2935
Lee XH, Kim K, Smith R (2007) Temporal variations of the 18O/16O signal of the whole-canopy transpiration in a temperate forest. Glob Biogeochem Cycles 21:Article Number GB3013
Lendzian KJ (2006) Survival strategies of plants during secondary growth: barrier properties of phellems and lenticels towards water, oxygen, and carbon dioxide. J Exp Bot 57:2535–2546
Li Y, Sperry JS, Taneda H, Bush SE, Hacke UG (2008) Evaluation of centrifugal methods for measuring xylem cavitation in conifers, diffuse- and ring-porous angiosperms. New Phytol 177:558–568
Lopez OR, Kursar TA (2003) Does flood tolerance explain tree species distribution in tropical seasonally flooded habitats? Oecologia 136:193–204
Lopez OR, Kursar TA, Cochard H, Tyree MT (2005) Interspecific variation in xylem vulnerability to cavitation among tropical tree and shrub species. Tree Physiol 25:1553–1562
Mathieu R, Bariac T (1996) A numerical model for the simulation of stable isotope profiles in drying soils. J Geophys Res Atmos 101:12685–12696
McCole AA, Stern LA (2007) Seasonal water use patterns of Juniperus ashei on the Edwards Plateau, Texas, based on stable isotopes in water. J Hydrol 342:238–248
McCulloh KA, Meinzer FC, Sperry JS, Lachenbruch B, Voelker SL, Woodruff DR, Domec JC (2011) Comparative hydraulic architecture of tropical tree species representing a range of successional stages and wood density. Oecologia 167:27–37
McDowell NG (2011) Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol 155:1051–1059
McDowell N et al (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739
McDowell NG, Beerling DJ, Breshears DD, Fisher RA, Raffa KF, Stitt M (2011) The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol Evol 26:523–532
McElrone AJ, Pockman WT, Martinez-Vilalta J, Jackson RB (2004) Variation in xylem structure and function in stems and roots of trees to 20 m depth. New Phytol 163:507–517
Penuelas J, Terradas J, Lloret F (2011) Solving the conundrum of plant species coexistence: water in space and time matters most. New Phytol 189:5–8
Pittermann J, Sperry JS, Wheeler JK, Hacke UG, Sikkema EH (2006) Mechanical reinforcement of tracheids compromises the hydraulic efficiency of conifer xylem. Plant Cell Environ 29:1618–1628
Reu B et al (2011) The role of climate and plant functional trade-offs in shaping global biome and biodiversity patterns. Glob Ecol Biogeogr 20:570–581
Romm J (2011) The next dust bowl. Nature 478:450–451
Russell FL, Fowler NL (1999) Rarity of oak saplings in savannas and woodlands of the eastern Edwards Plateau, Texas. Southwest Nat 44:31–41
Sala A, Piper F, Hoch G (2010) Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol 186:274–281
Schenk HJ (2008) Soil depth, plant rooting strategies and species’ niches. New Phytol 178:223–225
Schulze ED et al (1996) Rooting depth, water availability, and vegetation cover along an aridity gradient in Patagonia. Oecologia 108:503–511
Schwinning S (2008) The water relations of two evergreen tree species in a karst savanna. Oecologia 158:373–383
Schwinning S, Ehleringer JR (2001) Water use trade-offs and optimal adaptations to pulse-driven arid ecosystems. J Ecol 89:464–480
Shinneman DJ, Baker WL (2009) Historical fire and multidecadal drought as context for pinon-juniper woodland restoration in western Colorado. Ecol Appl 19:1231–1245
Sperry JS (2000) Hydraulic constraints on plant gas exchange. Agric For Meteorol 104:13–23
Sperry JS, Saliendra NZ (1994) Intra-plant and inter-plant variation in xylem cavitation in Betula occidentalis. Plant Cell Environ 17:1233–1241
Sperry JS, Tyree MT (1990) Water-stress induced xylem embolism in 3 species of conifers. Plant Cell Environ 13:427–436
Sperry JS, Donnelly JR, Tyree MT (1988) Seasonal occurrence of xylem embolism in sugar maple (Acer saccharum). Am J Bot 75:1212–1218
Sperry JS, Adler FR, Campbell GS, Comstock JP (1998) Limitation of plant water use by rhizosphere and xylem conductance: results from a model. Plant Cell Environ 21:347–359
Sperry JS, Meinzer FC, McCulloh KA (2008) Safety and efficiency conflicts in hydraulic architecture: scaling from tissues to trees. Plant Cell Environ 31:632–645
Taneda H, Sperry JS (2008) A case-study of water transport in co-occurring ring- versus diffuse-porous trees: contrasts in water-status, conducting capacity, cavitation and vessel refilling. Tree Physiol 28:1641–1651
Van Auken OW, Ford AL, Stein A, Stein AG (1980) Woody vegetation of upland plant communities in the southern Edwards Plateau. Tex J Sci 32:23–35
West AG, Hultine KR, Burtch KG, Ehleringer JR (2007) Seasonal variations in moisture use in a piñon-juniper woodland. Oecologia 153:787–798
Wills FH (2005) Structure of historic vegetation on Kerr Wildlife Management Area, Kerr County, Texas. Tex J Sci 57:137–152
Willson CJ, Jackson RB (2006) Xylem cavitation caused by drought and freezing stress in four co-occurring Juniperus species. Physiol Plant 127:374–382
Willson CJ, Manos PS, Jackson RB (2008) Hydraulic traits are influenced by phylogenetic history in the drought-resistant, invasive genus Juniperus (Cupressaceae). Am J Bot 95:299–314
Wrede J (2005) Trees, shrubs, and vines of the Texas Hill Country. Texas A&M University Press, College Station
Acknowledgments
We thank Georgianne Moore for technical advice on sap flow sensor construction, implementation, and analysis. We thank Heather Cardella Dammeyer for field assistance and Jim and Shannon Brotherton for allowing access to their land. Funding was provided by the Texas Higher Education Coordinating Board, Norman Hackerman—Advanced Research Program Grant (Project # 003615-0021-2007) and the Howard McCarley Student Research Award of the Southwestern Association of Naturalists.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Hermann Heilmeier.
Rights and permissions
About this article
Cite this article
Kukowski, K.R., Schwinning, S. & Schwartz, B.F. Hydraulic responses to extreme drought conditions in three co-dominant tree species in shallow soil over bedrock. Oecologia 171, 819–830 (2013). https://doi.org/10.1007/s00442-012-2466-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2466-x