Abstract
Previous tests using a growth medium and olive mill wastewater (OMWW) have shown that it supplies carbon and electron donors suitable for sulphate reducing bacteria (SRB). We assessed the co-treatment of acid mine drainage (AMD) and OMWW using SRB-enriched bioreactors and identified the most abundant bacterial populations present under optimized conditions. The process requires a neutralizing agent to create optimal pH conditions for successful removal of the AMD’s main contaminants. Concentrations of SO42−, Al, Fe, Cu, Zn, and Mn decreased to below Portugal’s maximum admissible values for irrigation waters, and all but Mn were reduced to less than Portugal’s emission limit values (ELVs) for wastewater discharges. Phenol concentrations—the main pollutants in OMWW—dropped to values between 1/10 and 1/5 their initial concentrations in batch tests using mixtures of AMD and OMWW, and to 1/2 their initial concentrations in flow-through tests. The final total phenol concentrations were still above the ELV for wastewater discharges, but phenols are not regulated in irrigation waters, and OMWW is used by some producers to irrigate soils. Six main SRB groups were identified as likely having a fundamental role in the bioremediation process: the genera Desulfovibrio, Sulfurospirillum, and Acetobacter and the families Sphingomonadaceae, Prevotellaceae, and Deferribacteraceae.
Zusammenfassung
Frühere Tests mit einem Wachstumsmedium und Olivenmühlenabwasser (OMWW) haben gezeigt, dass es Kohlenstoff- und Elektronenspender liefert, die für sulfatreduzierende Bakterien (SRB) geeignet sind. Wir untersuchten die gemeinsame Behandlung von sauren Grubenwässern (AMD) und OMWW mit SRB-angereicherten Bioreaktoren und identifizierten die am häufigsten vorkommenden Bakterienpopulationen unter optimierten Bedingungen. Der Prozess erfordert ein Neutralisationsmittel, um optimale pH-Bedingungen für eine erfolgreiche Entfernung der Hauptschadstoffe der AMD zu schaffen. Die Konzentrationen von SO42−, Al, Fe, Cu, Zn und Mn sanken unter die in Portugal zulässigen Höchstwerte für Bewässerungswasser, und alle außer Mn wurden unter die portugiesischen Emissionsgrenzwerte (ELVs) für Abwassereinleitungen reduziert. Die Phenolkonzentrationen—die wichtigsten Schadstoffe in OMWW—sanken auf Werte zwischen 1/10 und 1/5 ihrer Anfangskonzentrationen in Batch-Tests unter Verwendung von Mischungen aus AMD und OMWW und auf die Hälfte ihrer Anfangskonzentrationen in Durchflusstests. Die endgültigen Gesamtphenolkonzentrationen lagen immer noch über dem ELV für Abwassereinleitungen, aber Phenole werden in Bewässerungswasser nicht reguliert, und OMWW werden von einigen Produzenten zur Bewässerung von Böden verwendet. Es wurden sechs SRB-Hauptgruppen identifiziert, die wahrscheinlich eine grundlegende Rolle im Bioremediationsprozess spielen: Die Gattungen Desulfovibrio, Sulfurospirillum, und Acetobacter sowie die Familien Sphingomonadaceae, Prevotellaceae und Deferribacteraceae.
Resumen
En pruebas anteriores donde se utilizaba un medio de crecimiento y aguas residuales de molino de aceite (OMWW), se ha demostrado que permitía el crecimiento de bacterias reductoras de sulfatos (SRB) ya que suministra carbono y donantes de electrones adecuados. En este trabajo se evaluó el co-tratamiento del drenaje ácido de mina (AMD) y OMWW utilizando biorreactores enriquecidos con SRB e identificamos las poblaciones bacterianas más abundantes presentes en condiciones optimizadas. El proceso requiere un agente neutralizante para crear condiciones de pH óptimas para la eliminación satisfactoria de los principales contaminantes del DMA. Las concentraciones de SO42−, Al, Fe, Cu, Zn y Mn disminuyeron por debajo de los valores máximos admisibles en Portugal para las aguas de regadío, y todas, excepto el Mn, se redujeron a menos de los valores límite de emisión (VLE) en Portugal para las descargas de aguas residuales. Las concentraciones de fenol -los principales contaminantes en OMWW- disminuyeron a valores entre 1/10 y 1/5 de sus concentraciones iniciales en pruebas en batch utilizando mezclas de AMD y OMWW, y a 1/2 de sus concentraciones iniciales en pruebas de flujo. Las concentraciones finales totales de fenoles seguían siendo superiores al VLE de las descargas de aguas residuales, pero los fenoles no están regulados en las aguas de riego, y OMWW es utilizado por algunos productores para regar los suelos. Se identificaron seis grupos principales de SRB que probablemente desempeñen un papel fundamental en el proceso de biorremediación: los géneros Desulfovibrio, Sulfurospirillum, y Acetobacter y las familias Sphingomonadaceae, Prevotellaceae y Deferribacteraceae.
抽象
以往实验已经表明, 培养基和橄榄加工厂废水(OMWW)可以为硫酸盐还原菌(SRB)提供理想碳源和电子供体。研究评价了用富含硫酸盐还原菌(SRB)的生物反应器合并处理矿山酸性废水(AMD)和橄榄加工厂废水(OMWW)的可行性, 识别了最优化处理条件下的最大生物菌群。废水处理过程需要用一种中和剂创造出最佳pH环境, 以成功去除酸性废水主要污染物。处理后, SO42−, Al, Fe, Cu, Zn和Mn浓度已经低于葡萄牙灌溉用水的最大允许值, 除了Mn以外其它成分也都降到了葡萄牙废水排放限值标准(ELVs)以下。苯酚是橄榄加工厂废水(OMWW) 的主要污染物, 浓度已经下降到矿山酸性废水(AMD)和橄榄加工厂废水(OMWW)混合物批次试验初始浓度的1/10到1/5之间和穿透渗流试验初始浓度的1/2。虽然苯酚最终总浓度仍然高于废水排放限值标准(ELV), 但是由于灌溉用水没有酚类设限规定, 一些生产商会用橄榄加工厂废水(OMWW)灌溉农田。最终确认的六种可能在生物修复中发挥主要作用的硫酸盐还原菌(SRB)群落:脱硫弧菌属(Desulfovibrio), 硫螺菌属(Sulfurospirillum,)和乙酰杆菌属(Acetobacter)及鞘孢单胞菌科(Sphingomonadaceae), 普雷沃氏菌科(Prevotellaceae)和铁胆杆菌科(Deferribacteraceae)。
Similar content being viewed by others
Introduction
Acid mine drainage (AMD), also known as acid rock drainage, can contain high concentrations of sulphate, metals, and metalloids that can contaminate groundwater and watercourses and damage the health of aquatic species, plants, wildlife, and humans (Johnson 2003; Simate and Ndlovu 2014). The main cause of AMD is the oxidation of sulphide minerals (mainly pyrite) due to their exposure to oxygen, water, and microorganisms. It may occur naturally but is accelerated by mining activities that increase the exposure of Fe sulphide minerals to such conditions (Egiebor and Oni 2007; Johnson 2003; Johnson and Hallberg 2005).
AMD treatment approaches can be divided into those that rely on biological activities (biotic) and those that do not (abiotic), and can be classified into three broad categories—active, passive, and semi-passive treatment. Active treatment requires significant ongoing costs for operation, including labour, chemicals, and electricity, and are thus generally more appropriate for use at operating mines or for high flow rates, while passive and semi-passive treatment are more cost-effective solutions for inactive or abandoned mines sites where the remote location or other factors require the use of low-maintenance, low-cost treatment options (Johnson and Hallberg 2005; Simate and Ndlovu 2014; Skousen et al. 2017). Passive systems can operate for a long time (typically at least a decade) without any supplemental chemicals or energy, while semi-passive systems require a low dose of chemicals or nutrients, but without the need of continuous labour and power.
Abiotic passive treatment methods include geochemical systems such as open limestone channels, limestone leach beds, limestone sand, anoxic limestone drains, diversion wells, steel slag leach beds, and low-pH Fe oxidation channels (Kefeni et al. 2017; Kleinmann et al. 1998; Skousen et al. 2017; Valls and de Lorenzo 2002). Biological treatment methods have been viewed with considerable interest because metals from AMD are removed in natural wetland ecosystems. Since natural treatment of AMD was found in Sphagnum moss dominated bogs, the early systems were attempts to simulate such systems (e.g. Kleinmann et al. 1983). However, they were effective only for sites with relatively low metal loads and at most sites, the moss rapidly died (Gazea et al. 1996). Despite this, the research on using wetlands continued and eventually a wetland design evolved that proved tolerant to AMD and effective at reducing the levels of dissolved metals (Kleinmann and Hedin 1993). Most of these passive biological AMD treatment systems consist of a series of constructed ponds amended with organic substrates and planted with cattails, sedges, and rushes to resemble natural wetlands (e.g. Girts et al. 1987). In such systems, Mn- and Fe-oxidizing microbes precipitate these metals as oxides and contribute to the coprecipitation of other metals and metal hydroxides (Gazea et al. 1996). This works well for most coal mine drainage, but metal mines typically require the activity of sulphate reducing bacteria (SRB), which reduce sulphate to sulphide and forms metal sulphide precipitates (Cohen 2006). SRB require strict anaerobic environments with a pH in the range of 5–8, and if the pH and/or redox conditions are not ideal, microbial sulphate reduction declines and metal removal capacity is impaired (e.g. Brown et al. 1973). Over time, improved biological remediation techniques were developed using a variety of constructed compost and sulfidogenic bioreactors, in combination with abiotic methods such as the addition of limestone or other alkaline materials (Boonstra et al. 1999; Johnson and Hallberg 2005; Maree and Hill 1987; Skousen et al. 2017). Aerobic wetlands, packed-bed Fe oxidation bioreactors, and compost bioreactors are considered passive biological systems, while off-line sulfidogenic bioreactors are considered active biological systems (Johnson and Hallberg 2005; Simate and Ndlovu 2014; Skousen et al. 2017).
There are also many examples of semi-passive systems where the AMD is at least partially treated by more acid-tolerant SRB and by either a continual or flow-regulated addition of suitable liquid organic wastes rich in carbon sources and/or electron donors (e.g. lactate, ethanol, sucrose; URS 2003).
In passive biotic systems, the most efficient organic materials are mixtures of substrates that contain easily biodegradable compounds (soluble sugars, starch, and proteins) and promote the rapid establishment of microbial populations with SRB communities. Solid, less-labile substrates (with cellulose, hemicelluloses, and lignin) will also promote long-term SRB activity based on the use of products generated from their slow degradation. In contrast, in active and semi-passive biotic systems, where rapid treatment of large amounts of water is needed, the use of pure or easily degradable nutrient sources is typically more appropriate (Neculita et al. 2007; Sheoran et al. 2010; Skousen et al. 2017).
In the olive oil industry, both the traditional press extraction method and the continuous three-phase decanter process generate three products: olive oil (~ 20%) and two streams of waste: the solid crude olive cake or olive husk (≈30%) and the aqueous olive mill wastewater (OMWW) or olive mill effluent (≈50%) (Tsagaraki et al. 2007). Approximately 3.1 × 106 t of olive oil are produced annually worldwide, with more than 95% of it produced in the Mediterranean region (FAOSTAT 2014). With an average of 0.2 t of olive oil extracted per t of processed olives and an average of 1.2 m3 OMWW produced per t of milled olives (Amaral 2009; Jeguirim et al. 2017), the estimated annual worldwide production of OMWW is 18.6 × 106 m3. OMWW has a high chemical oxygen demand (COD) due to a considerable content of sugars and its high phosphorus content, which can be easily treated by biological processes. However, recalcitrant phenolic compounds present in OMWW impart toxic characteristics to this effluent (Capasso et al. 1995; Niaounakis and Halvadakis 2006; Sayadi et al. 2000). The most common phenols in OMWW are polyphenols of different molecular mass; the list of specific phenols in OMWW is complex and variable, depending on several factors (e.g. climatic conditions and storage time of olives) (Obied et al. 2005). The discharge of raw OMWW into lakes and rivers is forbidden in all European Union countries due to its harmful effects on the ecological balance. Therefore, ponds are usually constructed to promote evaporation during summer, but they can cause a severe odour nuisance (Koutsos et al. 2018). In addition, in many cases, when the ponds are full, they can no longer accept OMWW, and olive oil production must cease (Renato Rocha, owner of a small/medium olive oil mill, personal communication, Nov. 2016). Due to the financial effects of this on producers, illegal disposal of untreated OMWW into aquatic resources has been observed (e.g. Elhag et al. 2017).
Recently, OMWW was successfully tested as a source of carbon and electron donors for SRB in a growth medium lacking any other organic compound, opening the way for further studies to evaluate the use of this waste in SRB-based processes to treat AMD (Carlier et al. 2019). The purpose of this study was to test a process for AMD treatment that combines an abiotic passive step using alkaline materials with a semi-passive biotic step. The biotic step was comprised of an SRB-based bioreactor that uses OMWW as a source of carbon and electron donors. The potential of the process to co-treat both wastewaters was evaluated. In addition, the taxonomic composition of bacterial communities at the end of the batch tests and in the optimized continuous bioreactor was analysed with the goal of identifying the major bacterial groups that are important to the process.
Materials and Methods
Source of AMD
The AMD used in this work was collected at the São Domingos Mine (37° 40′06.7″ N 7° 29′28.5″ W), in southeast Portugal, near the Spanish border. The mine is located in the Iberian pyrite belt (IPB), which extends along the southern region of Portugal to Rio Tinto in Spain and is considered one of the largest metallogenetic provinces of volcanogenic massive sulphide deposits in the world (Alvarenga et al. 2012; Álvarez-Valero et al. 2008). The São Domingos Mine was exploited during the Roman and Islamic occupations of the Iberian Peninsula and was the largest mine operating in Europe between 1857 and 1966; massive pyrite was the main mineral ore exploited, and copper and sulphur were the main elements extracted (Tavares 2008). The mine has been abandoned since the 1960s, leading to serious environmental deterioration of the area. The Portuguese public company Empresa de Desenvolvimento Mineiro (EDM, S.A.) is responsible for the environmental rehabilitation of the mining area. However, as of 2013 most of the work has not been completed (Dias-Sardinha et al. 2013), and it remains incomplete. In the mine area, there is a deep open pit, old mining structures in a high level of decay, ruins of industrial buildings, and tons of mining generating AMD contaminated with sulphate and metals (mainly Al, Fe, Cu, Zn, and Mn) that flows through characteristic reddish-yellow diverting channels with several dams (Pereira et al. 2004). These channels discharge to the Mosteirão stream, which then enters into the Chança River, a major tributary of the Guadiana River. The AMD from São Domingos is highly acidic (≈ pH 2) and has the following approximate constituent concentration ranges: sulphate (1000–5000 mg/L), Al (100–500 mg/L), Fe (50–500 mg/L), Zn (20–150 mg/L), Cu (20–100 mg/L), and Mn (5–20 mg/L) (e.g. Costa and Duarte 2005; Costa et al. 2008). The AMD samples used in this work were collected in the winter (January 31) and summer (July 27) of 2015 from one of the reddish-yellow dams (Online Resource, Fig. OR1) and were immediately transported (within ≈ 90 min) to the laboratory for characterization (Table 1).
Source of OMWW
The OMWW used in this study was collected in autumn 2015 from the mill Lagar de Santa Catarina (37° 09′05.8″ N 7° 47′20.5″ W), located in the village Santa Catarina da Fonte do Bispo, Algarve, Portugal. The collection of OMWW for this work was done in the first of four successive settling tanks (≈10,000 L each) downstream from the separation of olive oil and crude olive cake, and upstream of the final evaporation pond (Online Resource, Fig. OR2). An initial characterization of the OMWW was conducted by analysing a sample for pH, COD, total nitrogen, total phosphate, sulphate, and total phenols (Table 2).
Source and Cultivation of the SRB Community
The seeding community of SRB used for this work was obtained from a sludge sample collected from a wastewater treatment plant located between Faro and Olhão, in southern Portugal, by enrichment in Postgate B medium (Postgate 1984) under anaerobic conditions at room temperature (25 ± 3 °C) for about 2 weeks. The SRB community was maintained in the laboratory under these conditions through successive cultures inoculated with 1% (v/v) of the previous ones.
Batch Reactor Experiments
Neutralization of AMD for the Batch Tests
The seeding SRB communities used in this work are active under neutral conditions but are less active at pH values < 5.5 (Martins et al. 2009). Therefore, the pH of the winter AMD (wAMD) used in the batch tests was first neutralized using limestone gravel (grain size 0.5–1.5 cm) purchased from LUSICAL, a company of the Lhoist group (https://www.lhoist.com/), at a ratio of 1 kg/L of AMD in an overnight (16 h) bath. The effect on metal concentrations and pH will be discussed later. The liquid fraction was then decanted and used for the batch tests.
Experimental Setup for the Batch Tests
In previous work, a test using a 20% (v/v) dose of OMWW in Postgate B medium without lactate (making a 1:5 dilution) revealed high SRB activity (Carlier et al. 2019). Therefore, in this work the batch experiments were performed using the same dilution factor. The batch tests were performed using 20% (v/v) OMWW diluted in pH-neutralized winter AMD (nwAMD), with 5% (v/v) inoculum of the seeding SRB-enriched culture. The positive controls (no nwAMD) consisted of 20% (v/v) OMWW diluted in Postgate B without lactate, inoculated with 5% (v/v) of the same seeding SRB-enriched culture used in the tests. The negative controls (no SRB) consisted of batch flasks with 20% (v/v) OMWW (previously centrifuged to remove bacteria) diluted in either Postgate B medium without lactate or in nwAMD.
In a first set of 21 day batch tests, the mixture of the acidic OMWW with nwAMD became too acidic (pH 4.28), preventing the rapid growth of SRB. Therefore, a second set of batch tests kept for 28 days was carried out in which 10% (w/v) of a fine powder residue from a marble stone cutting and polishing industry was added to the cultures as a buffering agent. This ground marble is mainly composed of dolomite (*89%), quartz (*11%), and traces of illite (Barros et al. 2009), and previous tests showed that no biological sulphate reduction occurs in the presence of this material without the addition of a suitable carbon source/electron donor (data not shown). Table 3 summarizes the experiments performed on the batch reactors.
The assays were all carried out in triplicate using 100 mL glass bottles. The medium was purged with nitrogen gas to create anoxic conditions, and about 10 mL of liquid paraffin was added to eliminate oxygen diffusion. The bottles were sealed with butyl rubber stoppers and aluminium crimp seals and incubated at room temperature (25 ± 3 °C). Postgate B medium, glass bottles, rubber stoppers, and pipetting materials used for the batch experiments were previously autoclaved for 20 min at 121–124 °C (200 kPa) for sterilization.
Monitoring of the Batch Tests
To monitor the evolution of the batch reactors, 2 mL samples were collected from the initial media and from all replicates during the experiments through the rubber stoppers using a syringe and analysed. The maximum total volume collected was 10 mL (10% of the initial volume). Dissolved sulphate and sulphide concentrations were monitored weekly as indicators of SRB activity, while redox potential (Eh) and pH were monitored weekly due to their importance as limiting factors for SRB growth (O’Flaherty et al. 1998; Postgate 1984; Willow and Cohen 2003). The concentrations of dissolved Fe, Cu, and Zn were determined weekly to evaluate metal removal in the incubation period. The concentration of total phenols was determined in the initial and final samples.
Continuous Flow System Experiments
Continuous Flow System
The continuous flow bioremediation tests for AMD treatment using a SRB-based process feed with OMWW as a carbon source/electron donor supplement were carried out in a system comprised of two main components: a neutralization tank with limestone gravel, and an upflow anaerobic packed bed (UAPB) bioreactor enriched with SRB (Fig. 1). The glass neutralization tank was 22 cm long, 15 cm wide, and 7 cm deep, had 1 kg of crushed limestone (grain size: 0.5–1.5 cm) arranged so that the AMD passed through the limestone particles (see the profile section of the neutralization tank in Fig. 1), and had a working volume of ≈ 1 L. The UAPB bioreactor was a 35 cm high and 5.5 cm diameter glass cylinder filled initially with coarse sand (grain size: 0.2–0.5 cm); to correct the observed acidification, it was subsequently filled with a mixture of coarse sand and crushed limestone in a 1/1 ratio (v/v) to maintain a working volume of ≈ 400 mL. The bioreactor and the container collecting the effluent were placed inside a fume hood to prevent contamination of laboratory air with H2S.
Start-Up and Acclimatization Stages of the UAPB Bioreactor
The bioreactors were enriched with SRB for 2 weeks in batch mode using Postgate B medium inoculated with 10% (v/v) of the same seeding SRB culture used to inoculate the batch tests. After this start-up period, after sulphate reduction was observed by decay of ≈90% of the sulphate concentration and corresponding rise in sulphide, the most probable number method was applied using anaerobic test tubes with Postgate E medium to confirm that the number of SRB colony-forming units (CFU) reached at least 106. Afterwards, to acclimate the microbial community to AMD and to OMWW, the flow was initiated at 2.5 mL/h, making a hydraulic retention time (HRT) of 6.7 days in the bioreactor: first, for 5 days, the bioreactor was fed a mixture of 60% (v/v) Postgate B, 40% (v/v) nwAMD (delivered from the neutralization tank), and supplements of 0.4 mL ethanol injected in the bottom of the bioreactor at 2-day intervals to compensate for the lack of carbon sources in the AMD; thereafter, the bioreactor was fed a mixture of 35% Postgate B, 45% nwAMD, and 20% OMWW until high sulphate reduction activity was restored. Both the start-up and acclimatization stages were conducted at room temperature (25 ± 3 °C).
Treatment Tests on the UAPB Bioreactor
After the start-up and acclimatization stages, the stock of wAMD was depleted, and it was necessary to collect additional AMD during the summer (sAMD) for the continuous flow treatment tests (Table 1). The continuous treatment tests were also performed at room temperature (25 ± 3 °C) and began with a mixture of 80% (v/v) sAMD and 20% (v/v) OMWW (the dilution successfully tested in the batch experiments), with the same flow rate and HRT used in the acclimatization stage (flow = 2.5 mL/h; HRT = 6.7 days). Several operational conditions were tested for process optimization, as described in the results and discussion section.
Monitoring the UAPB Bioreactor
To monitor the continuous flow system during the start-up and acclimatization stages, pH, Eh, and sulphate and sulphide concentrations were determined in the neutralization tank outlet and in the bioreactor outlet, approximately weekly. During the treatment tests phase, these four parameters and Al, Fe, Zn, Cu, and Mn concentrations were determined in both outlets on the same frequency. Total phenol concentration was determined in the bioreactor outlet, though less frequently (18 day intervals, on average).
Analytical Methods
Samples from the batch and the flow-through tests were analysed after centrifugation at 2500 g for 5 min at room temperature to remove suspended solids. A pH/E Meter GLP 21 (CRISON) was used to measure Eh with a Pt electrode coupled with a reference-saturated calomel electrode (CRISON, 52 61) and pH with a glass pH electrode (VWR, SJ 223). Redox measurements were converted to Eh values using a conversion factor of 241 mV for the Pt electrode. A UV–visible spectrometer DR2800 (Hach-Lange) was used to determine: (1) the sulphate and sulphide concentrations, using the sulfaVer4 (Method 8051, Hach-Lange) and the methylene blue (Method 8131, Hach-Lange) procedures, respectively at 450 and 665 nm; (2) COD, using the dichromate method (kit LCK 514, Hach-Lange) with 2 h digestion at 148 °C, at 605 nm; (3) total nitrogen and total phosphorus, using persulphate digestion (Method 10,072, Hach-Lange) and molybdovanadate with acid persulphate digestion (Method 10,127, Hach-Lange) procedures, respectively at 410 and 420 nm; (4) total phenols, using the 4-nitroaniline method (Kits: LCK 345 (0.05–5 mg/L) and LCK 346 (5–200 mg/L) Hach-Lange, respectively at 478 and 510 nm). Metals determination was conducted after acidification of the samples with nitric acid (to 5%). The concentrations of Fe, Zn, Cu, and Mn were measured using flame atomic absorption spectroscopy with a novAA 350 system (Analytik Jena), and the concentration of aluminium was measured by microwave plasma atomic emission spectrometry with a 4200 MP-AES (Agilent), in both cases with calibration curves developed using standards prepared from the following stock solutions: Fe(NO3)3 in 0.5 M HNO3, Zn(NO3)2 in 0.5 M HNO3, and Cu(NO3)2 in 0.5 M HNO3 (Merck Certipur, Germany); Mn(NO3)2 in 0.5 M HNO3 and Al(NO3)3 in 0.5 M HNO3 (Panreac AA, Spain).
Taxonomic Characterization of Bacterial Communities
Studied Communities
To identify the most important bacteria involved in the tested treatment process, the bacterial population composition was studied in the SRB-enriched culture used as an inoculum, in two samples from the batch experiments, and in one sample from the continuous UAPB bioreactor. Samples from the batch experiments were collected at the end of both tests with 20% OMWW, 75% nwAMD, and 5% SRB inoculum: one with the 10 g supplement of marble powder as a buffering material (sample name: Y) and the other without the marble powder (sample name: AR). The UAPB bioreactor sample used for bacterial population studies was collected on operational day 372, during the last week of this experiment (sample name: Reactor IV).
DNA Extraction
The DNA extraction was accomplished by centrifuging 10-mL samples at 2500 g for 10 min to collect cells. Supernatants were discarded, and the cells were resuspended in 200-µL TEG buffer (25 mM TRIS, 10 mM EDTA pH 8.0 and 50 mM glucose). Genomic DNA was subsequently extracted and purified using the PowerSoil DNA Isolation Kit (MO BIO laboratories) according to the kit’s protocol.
16S rRNA Amplicon Library Preparation
The extracted and purified DNA was used for library construction of the V1-V3 16S rRNA gene’s region by polymerase chain reaction (PCR) based on Caporaso et al. (2012) and using primers (27F: AGAGTTTGATCCTGGCTCAG and 534R: ATTACCGCGGCTGCTGG) adapted from the Human Gut Consortium (Ward et al. 2012). PCR amplifications were performed using reactions of 25 µL, with 10 ng of extracted DNA as the template, 400 µM of each dNTP, 1.5 mM of MgSO4, 1 U of Platinum®Taq DNA polymerase HF, in 1X Platinum® High Fidelity buffer (Thermo Fisher Scientific, USA), and 400 nM of each primer containing barcoded library adaptors. The settings for PCR cycling were: one initial denaturation step at 95 °C for 2 min, 30 cycles of amplification (95 °C for 20 s, 56 °C for 30 s, 72 °C for 60 s), and a final elongation step at 72 °C for 5 min. Duplicate PCR amplifications were performed for each sample, and the duplicates were pooled. The amplicon libraries were purified using the standard protocol for Agencourt Ampure XP Bead (Beckman Coulter, USA) with a modified bead to sample ratio of 4:5, then eluted in 33 μL of nuclease-free water (Qiagen, Germany); their DNA concentration was measured using Quant-iT™ HS DNA Assay (Thermo Fisher Scientific, USA) and quality validated with a Tapestation 2200, using D1000 ScreenTapes (Agilent, USA).
DNA Sequencing
The purified sequencing libraries were pooled in equimolar concentrations and diluted to 4 nM. The samples were paired end sequenced (2 × 301 base pairs) on a MiSeq (Illumina) using a MiSeq Reagent kit v3 (Illumina, USA) following the kit’s standard guidelines for preparing and loading samples on the MiSeq. The Phix control library was used as an in-run control (20% spiked-in) for run quality monitoring to overcome the low complexity issue often observed with amplicon samples.
16S rRNA Amplicon Bioinformatic Processing
Initial processing included trimming the forward and reverse sequences for quality reads using Trimmomatic v. 0.32 (Bolger et al. 2014) with the settings SLIDINGWINDOW:5:3 and MINLEN:275. The trimmed forward and reverse reads were subsequently merged using FLASH v. 1.2.7 (Magoc and Salzberg 2011) with the settings -m 25 -M 200, and the merged sequences were dereplicated and formatted using the UPARSE workflow (Edgar 2013). The dereplicated reads were then clustered into operational taxonomic units (OTUs) using the “cluster_otus” command of USEARCH (vers. 7.0.1090; Edgar 2013) with default settings, and the OTU abundances were estimated for a 97% sequence identity using the “usearch_global” command with parameter-id 0.97. The taxonomic classifications of OTUs were assigned using the RDP classifier (Wang et al. 2007) as implemented in the parallel_assign_taxonomy_rdp.py script in QIIME (Caporaso et al. 2010), using the Greengenes database taxonomy v.13.8. The results were analysed in R (R Core Team 2015) through the Rstudio IDE using the “ampvis” package v.1.9.1 (Albertsen et al. 2015).
Nucleotide Sequence Accession Numbers
The DNA sequences obtained from the batch tests were published in the NCBI high-throughput DNA and RNA sequence read archive (SRA) with the identifiers BioProject: PRJNA304303 and SRA: SRP066901. The sequences from the test with 10% (w/v) marble powder (sample Y) were identified with BioSample: SAMN04297453; Runs: SRR2969424 and SRR2969423. The sequences from the test without marble powder (sample AR) were identified with BioSample: SAMN04297454; Runs: SRR2969430 and SRR2969425. The OTU sequences obtained from the taxonomic study in the batch experiments were published with GenBank accession numbers KU206784 to KU207047.
The DNA sequences obtained from the UAPB bioreactor (sample Reactor IV) have the identifiers BioProject: PRJNA472456, SRA: SRP148642, BioSample: SAMN09236008, and Run: SRR7195056. The OTU sequences obtained from the taxonomic study in the UAPB bioreactor were published with GenBank accession numbers MG554745 to MG557554.
Results and Discussion
Batch Reactors
Neutralization of AMD for the Batch Tests
The batch experiments were carried out with nwAMD (the pH of the wAMD increased from 2.48 to 6.32, causing metals to precipitate as metals hydroxides. Although the concentration of Fe in the nwAMD was low (0.07 mg/L), the concentrations of Zn and Cu were still relatively high (35 and 13 mg/L, respectively), as expected because precipitation of these elements occurs at higher pH values (Online Resource, Fig. OR3 to OR7). The sulphate concentration of the nwAMD remained as high (1803 mg/L) as in wAMD before pH neutralization (Table 1). These results are similar to those obtained by Vitor et al. (2015), who also used marble powder to neutralize AMD collected at the São Domingos Mine: the pH was raised to ≈ 7, and Fe was almost totally removed (to ≈ 0.1 mg/L), while Zn, Cu, and sulphate concentrations were respectively ≈ 10, 1, and 2000 mg/L.
Batch Reactors Without Marble Powder
After mixing the acidic OMWW (pH 4.13) with nwAMD (pH 6.32) without the addition of a buffering agent, the mixture became acidic (pH 4.28). Even though this initial acidity was not ideal for the activity and growth of SRB, during the first week of incubation, the pH rose to ≈ 6.8 and the sulphate concentration decreased at the end of the test to 53% of its initial value. Although the early literature claimed strict pH limits on SRB activity, subsequent studies have shown that sulphate reduction at pH values below 5 is possible (Koschorreck 2008). However, the maximum sulphide concentration measured during the experiment was just 3.3 mg/L, which is a sign of low SRB activity (Fig. 2a). In fact, the Eh fell below − 100 mV, which is known to be optimal for SRB (Postgate 1984), only in the last week of the experiment. Thus, it was not surprising that the final sulphate concentration was still relatively high (741 mg/L). Nevertheless, these results show that given time, favourable conditions for SRB were created in the batch reactors. In the negative control (no SRB; Fig. 2b), although sulphate decreased by 28%, the absence of sulphide production and high Eh (+ 217 to + 235 mV) indicate that this sulphate loss was not caused by SRB activity.
Results from the 21-day batch experiments without addition of marble residue powder: a Results from test with 20 mL OMWW, 75 mL nwAMD, and 5 mL SRB-enriched inoculum, b Results from negative control: 20 mL centrifuged OMWW, 75 mL nwAMD, no SRB enriched inoculum. A: sulphate, sulphide, and total phenols; B: pH and Eh; C: Zn, Fe, and Cu
With regard to metal removal, the assay with a SRB inoculum revealed promising results (99.4% Zn and 99.2% Cu removed) with final low concentrations for these metals: 0.20 mg/L Zn and 0.11 mg/L Cu (Fig. 2a). The role of biological activity in these removals was confirmed by the negative control (without SRB inoculum), in which Fe and Cu were not removed, and Zn decreased by only 29% (Fig. 2b).
The slight decreases in sulphate and Zn concentrations in the negative control could suggest the formation of zinc sulphate (ZnSO4). However, the decrease in both concentrations does not correspond to the expected stoichiometry for the mineral. There was a decrease of 5.21 mM of sulphate but only 0.16 mM Zn. It is more likely that there was some reduction of sulphate to sulphide, even if minimal, due to SRB putatively present in the OMWW, leading to precipitation of zinc sulphide (ZnS). Still, this does not justify the observed sulphate decrease. Another possibility is the precipitation of compounds or complexes resulting from some type of interaction between sulphate in the nwAMD and the phenols or other plant compounds present in the OMWW. Further research is needed to evaluate this hypothesis.
Regarding the concentration of total phenols, decreases to about one tenth of the initial value were observed after 3 weeks, both in the test inoculated with SRB and in negative control without inoculum (Fig. 2a, b). Even though the final concentration of total phenols in the inoculated and uninoculated flasks (5.5 mg/L and 5.0 mg/L, respectively) were still relatively high, the removal is important because phenols are the recalcitrant main pollutant in OMWW. The previous centrifugation of OMWW was expected to eliminate most suspended solids and bacteria but not to completely sterilize the wastewater. Therefore, it is likely that the OMWW contained trace amounts of phenol-degrading microbes that may have contributed to the observed decrease in concentration. Mineralisation of phenols can proceed under anaerobic conditions through different pathways metabolized by consortia of various microorganisms (Levén et al. 2012). Some abiotic reactions may have also contributed to the decrease in phenol concentrations. Information in the literature supports the possibility that some metals and/or the sulphate in the AMD may have helped degrade the phenols when the wastewaters were mixed. Several studies have shown that a wide range of environmental contaminants can be reduced by Fe2+ absorbed to or within Fe oxides; it has also been shown that zero-valent Fe or Fe2+ can generate reactive oxidants such as ·OH, Fe4+, and O2 to oxidize contaminants (e.g. Fang et al. 2013). Fenton reagents (oxidant hydrogen peroxide (H2O2) activated by several Fe2+-bearing minerals) producing the hydroxyl radical (·OH) are widely used as advanced oxidation processes for degrading recalcitrant organic contaminants. Another well-known oxidant widely used to break down organics is the permanganate ion (MnO4−), which has the advantages of being relatively cheap, easy, and safe to use, compared to hydrogen peroxide (e.g. de Souza e Silva et al. 2009). Moreover, it is known that the sulphate radical (SO4·−) is more powerful for the decomposition of contaminants at neutral pH than the hydroxyl radical (·OH). In fact, when peroxymonosulphate (SO52−) or peroxydisulphate (S2O82−) are activated by catalysts (such as Co, Ag, Cu, Fe), they generate SO4·−, which degrades organic pollutants (Mezyk et al. 2011; Zhang et al. 2015). Thus, sulphate radical-based advanced oxidation processes (SR-AOPs) using SO52− or S2O82− for the degradation of several organics, including phenols, have attracted increasing attention (e.g. Khan et al. 2017; Wang et al. 2015).
Batch Reactors with Marble Powder
In the second set of tests carried out with marble powder added to the batch flasks as a buffering material, the pH remained neutral (6.79–7.23), and a large reduction in sulphate concentrations was observed. In the inoculated test, the maximum SRB activity was observed during the second week, in which the Eh decreased from + 64 to − 313 mV, and 78% of the initial sulphate concentration was reduced (Fig. 3a). Throughout the test, sulphide reached a concentration of 160 mg/L and the sulphate concentration decreased from 1066 to 207 mg/L.
Results from the 28-day batch experiments with 10% (w/v) marble residue powder: a test with 20 mL OMWW, 75 mL nwAMD, and 5 mL SRB-enriched inoculum, b negative control with 20 mL centrifuged OMWW, 75 mL nwAMD, no SRB-enriched inoculum. A: sulphate, sulphide, and total phenols; B: pH and Eh; C: Zn, Fe, and Cu
The removal of metals by SRB was inconclusive because the pH neutralization before bacterial inoculation removed metals by precipitation, most likely as metal hydroxides. First, before the beginning of the assays, the wAMD was neutralized with limestone gravel to nwAMD (pH 2.48—> 6.32). Then, after the acidification caused by the addition of OMWW to nwAMD (pH 6.32—> 4.28), a second neutralization step occurred when marble powder was added to the batch flasks (pH 4.28—> 6.79). Nevertheless, three metals were detected in the beginning of the experiment (Cu: 1.14 mg/L; Zn 0.61 mg/L; Fe: 0.25 mg/L), and only one was above detection at the end (Cu < 0.4 mg/L; Zn < 0.07 mg/L; Fe: 0.53 mg/L) (Fig. 3a). In the negative control, the Eh remained positive (+ 68 to + 112 mV), which is not suitable for SRB, and no sulphate was removed. However, in the fourth week, a small increase in sulphide concentration was observed (Fig. 3b). This is a sign that some sulphate reduction occurred (though very low compared to the inoculated tests), which suggest the presence of SRB in the OMWW used in this work (despite the attempt to remove bacteria from it by centrifugation). Further work may be performed to discover whether these putative SRB belong to the species Desulfovibrio marrakechensis discovered in OMWW collected in Morocco by Chamkh et al. (2009) or if they belong to other species. The presence of sulphide in the negative control with marble powder justifies the decrease of Cu and Zn from 1.14 mg/L and 0.61 mg/L to 0.08 mg/L and 0.10 mg/L at the end of the experiment, respectively (Fig. 3b), suggesting possible precipitation as metal sulphides. The role of sulphide in these removals was confirmed by the negative control without marble powder, in which sulphide was not detected and Cu was not removed, while Zn decreased by just 29% (Fig. 2b).
Surprisingly, in both negative controls, the Fe concentration gradually increased during the experiments, reaching 4.98 mg/L without marble powder (Fig. 2b) and 2.30 mg/L with marble powder (Fig. 3b). In fact, even in both tests inoculated with SRB (with and without marble powder), the increasing trend in Fe concentrations was also observed in the first week, though values decreased as sulphate concentrations fell (Figs. 2a, 3a). It is known that Fe-based nanoparticles have a strong affinity for organic compounds, and several researchers have reported their use in removing various pollutants from wastewaters, including from OMWW (Nassar et al. 2014; Ochando-Pulido et al. 2013). It is possible that the increasing Fe concentration was caused by putative interactions between Fe-based particles and organic compounds from the OMWW.
Again, as in the experiment without marble powder, significant decreases in total phenols were observed in the test inoculated with SRB and the negative control without SRB. This reinforces the idea that this removal may have been caused by biological activity of microorganisms present in the OMWW and/or by abiotic processes, as discussed above. The final phenol concentrations (29.5 ± 0.8 mg/L and 31 ± 1 mg/L respectively) were about one fifth of the initial values in the batch flasks (Fig. 3a, b).
Continuous Flow System
Neutralization Tank
Effective neutralization of AMD from pH 2.48 (wAMD) or 2.28 (sAMD) to 7.2 ± 0.2 provided optimal pH conditions for SRB activity and growth (pH between 5 and 8; e.g. Cohen 2006). Moreover, also favouring these bacteria, the concentration of sulphate after the neutralization step remained high: during the acclimatization stages (when wAMD was used), the sulphate concentration in the neutralization tank outlet was 1668 ± 15 mg/L, while during the treatment tests (when sAMD was used), the sulphate concentration in the outlet of this tank was 2106 ± 96 mg/L (Fig. 4).
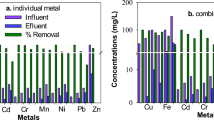
Regarding metals treatment in the neutralization tank, removal efficiencies > 99.5% were achieved for Al, Fe, and Cu, while removals of 32 ± 16% and 77 ± 5% were achieved for Mn and Zn, respectively (Fig. 4, Table 4). Manganese concentrations dropped from 14 ± 2 to 9 ± 1 mg/L after the hydraulic retention time (HRT) in the neutralization tank had been increased from 21 to 42 days by cutting the flow in half on the 246th day of the experiment. The flow of the entire system (neutralization tank and UAPB bioreactor) was adjusted depending on the sulphate reduction efficiency in the UAPB bioreactor, as discussed below.
The precipitation of metals in the neutralization step was visible as a fine orange sludge, but during the experiment, most of the sludge accumulated in the inlet zone of the tank where there was no limestone. Removal of metal ions in the neutralization tank was expected due to the higher pH, per the metal speciation-pH plots generated with Medusa-Hydra software (Puigdomenech 2015) (Online Resource, Fig. OR3-OR7). The diagrams show that Al and Fe begin to hydrolyse at low pH and form solid (oxy)hydroxides, while Cu and Zn hydrolyse and precipitate as hydroxide solids at still higher pH values. Mn will form oxides but not hydroxides at neutral and higher pH values. Abiotic Mn oxidation by oxygen is very slow at pH values below 9 (Stumm and Morgan 1995). Moreover, reduced Fe reacts faster with oxygen than Mn; its presence may inhibit or reverse Mn oxidation (Luan et al. 2012), and if Fe competes for oxygen, a pH of 10 may be needed to remove the Mn as an oxide (Lovett 1992). The higher HRT since day 246 probably allowed the water to stay in the neutralization tank for a longer time after Fe removal, thus decreasing the competition for oxygen and leading to slightly improved Mn removal. Indeed, Mn removal in limestone-filled channels at pH ≥ 7 proceeds much better if Fe and Al are first removed by pretreatment (Skousen et al. 2017).
Since the abiotic oxidation rate of Mn2+ is limited under neutral conditions and biotic oxidation processes are responsible for the formation of most environmental Mn oxides (Diem and Stumm 1984), the biological (microbial) oxidation of Mn has been considered as a viable alternative for water treatment removal of this metal (e.g. Katsoyiannis and Zouboulis 2004). Even if efficient bacterial generation of biogenic Mn oxide requires aeration and supplying organic nutrients, the use of algae can overcome this drawback since they are primary producers, and their photosynthesis increases dissolved oxygen levels and phycosphere pH, thus creating conditions for Mn oxidation (Wang et al. 2017). It has been long known that Mn precipitation can be achieved in passive in-line treatment systems with bacteria, cyanobacteria, fungi, diatoms, and green algae participating in the process (Robbins 1999).
UAPB Bioreactor
Sulphate removal efficiencies: The different operational conditions tested (Fig. 5) and the evolution of monitored parameters during the continuous flow experiment (Fig. 6) allowed us to evaluate and optimize the efficiency of the treatment process. During the bioreactor start-up in batch mode with Postgate B medium, from day 0 to 14, the decrease in sulphate concentration from 1781 to 97 mg/L and the achievement of 289 mg/L sulphide indicates high SRB activity and growth (Fig. 6a). This was confirmed by the high number of SRB CFUs (3.7 × 106) counted in a sample collected from the bioreactor midpoint at the end of this period (day 14). After the start-up period (at day 15), to acclimate the microbial community to AMD, the bioreactor was fed for 5 days with a 2.5 mL/h flow (making a HRT of 6.7 days) with a 60% (v/v) Postgate B and 40% (v/v) nwAMD mixture (see Fig. 5) and supplements of 0.4 mL ethanol injected at 2-day intervals to compensate for the lack of carbon sources in the AMD. During this period, high sulphate reduction was still observed, as shown by the 12 mg/L sulphate and 244 mg/L sulphide in the bioreactor outlet on day 19 (Fig. 6a). Then, to acclimate the microbial community to OMWW, starting on day 21, a mixture of 35% Postgate B, 45% nwAMD, and 20% OMWW was supplied at the same flow rate (2.5 mL/h) and HRT (6.7 days; see Fig. 5). This mixture caused a slight drop in pH (from day 0 until day 21, pH = 6.81 ± 0.08 and between days 36 and 46, pH = 6.53 ± 0.1) and a decreased rate of sulphate removal (on day 36, sulphate concentrations reached 1155 mg/L and sulphide dropped to 68 mg/L). This decline in sulphate reduction efficiency was expected because the microbial consortium in the bioreactor had been enriched and maintained with lactate- and ethanol-based feeds, compounds that can be directly used by most SRB, and then had to adapt to the carbon sources present in the OMWW, which must be first biologically transformed into simpler compounds. OMWW is an aqueous extract of olives that is rich in sugars (Amaral et al. 2008; Azbar et al. 2004) that, despite being soluble compounds, must first be transformed by fermenters into alcohols and organic acids that can be used by SRB (e.g. Seyler et al. 2003). Nevertheless, after that, SRB activity started to recover gradually: sulphate concentration in the bioreactor outlet decreased to 503 mg/L and sulphide reached 199 mg/L by day 57 and the pH rose to more neutral values (pH = 6.78 ± 0.12 between days 50 and 57).
Operational conditions in the UAPB bioreactor of the continuous flow experiment. Liquid medium/influent composition: Postgate B medium, OMWW, and neutralized AMD % (v/v). Neutralized winter AMD was used from day 1 to day 58; neutralized summer AMD was used from day 58 to end of the experiment. Packed bed composition: limestone gravel % (v/v) added to coarse sand. Hydraulic Retention Time (HRT) regime
After the start-up and acclimation stages, the continuous treatment tests began on day 58; the bioreactor was fed with a mixture of 80% nsAMD and 20% OMWW (see Fig. 5) with the same flow rate and HRT used in the acclimation stages (2.5 mL/h and 6.7 days). With this mixture, the pH remained neutral for 1 week but then started to drop gradually (Fig. 6a).
On day 90 (pH = 5.68), in a first attempt to reverse the acidification tendency, the bioreactor flow was decreased to 2 mL/h, resulting in a new HRT of 8.3 days. However, the pH continued to drop until it stabilized at a pH of 5.16 ± 0.04 between days 114 and 124), which is below the optimal range for SRB. Thus, as the pH dropped, sulphate concentrations rose (to 1508 mg/L) and sulphide concentrations decreased (to 25 mg/L) on day 124, indicating low SRB activity. This phenomenon has been previously reported for sugar-rich substrates. For example, Cao et al. (2012) observed a negative impact on the SRB growth and the sulphate removal rates when using sucrose, due to the acidity produced by fermentation, which resulted in high contents of volatile fatty acids (butanedioic, lactic, and formic acids). This acidification did not occur in the OMWW acclimation stage, likely because the lactate from the Postgate B was sufficient to assure direct sulphate reduction activity, and the alkaline compounds released from that activity helped maintain a neutral pH.
On day 124, in a second attempt to restore neutral pH, the amount of OMWW was intentionally decreased to 10% (half of the initial dose). Yet, after 1 week (on day 131) the pH in the bioreactor was still acidic (pH = 5.30), the sulphate concentration was high (1519 mg/L), and the sulphide concentration was low (31 mg/L).
In a third attempt to reverse the acidification trend, the amount of OMWW was decreased on day 132–5% (a quarter of the initial dose). After that, the pH began to rise gradually, becoming neutral on day 155 (pH = 6.91), improving SRB activity somewhat (sulphate concentrations dropped to 1052 mg/L and sulphide concentrations increased to 177 mg/L on day 204; Fig. 6a). In the following week, the concentrations of these compounds (1061 mg/L sulphate and 160 mg/L sulphide on day 211) indicated a steady-state process with incomplete sulphate reduction (50 ± 3% sulphate removal), most likely due to a lack of carbon sources/electron donors. When OMWW was tested for the first time as the only carbon source/electron donor using batch cultures with Postgate B medium without lactate, such low doses (4% and 3.5% OMWW) produced similarly low sulphate reduction efficiencies (55 ± 14% and 42 ± 2% sulphate removal rates were achieved, respectively; Carlier et al. 2019).
On day 211 the amount of OMWW was raised to the 20% dose successfully tested in the batch cultures, and the pH dropped again to acidic values (pH = 4.99 ± 0.04 between days 219 and 226) and a loss of SRB activity was again observed: sulphate concentrations increased to 1735 mg/L and sulphide concentration dropped to 5 mg/L on day 226 (Fig. 6a). This corresponds to a sulphate removal rate of just 23.1%, which confirmed the existence of a souring problem when OMWW is used to feed a bioreactor with SRB.
To prevent acidification, on day 229, the solid support bed for bacteria inside the bioreactor, which had just comprised coarse sand (grain size: 0.2–0.5 cm) was substituted by a 1/1 (v/v) mix of the same coarse sand plus crushed limestone (grain size: 0.5 to 1.5 cm; see Fig. 5). The experiment continued with a flow of 2 mL/h and an HRT of 8.3 days and an OMWW dose of 20%. After that, the bioreactor returned to neutral pH values (pH = 6.9 ± 0.4 from day 229 to the last day of operation), and SRB activity started to slowly recover. Sulphate removal was only 25.2% on day 246, sulphate concentrations dropped to 1666 mg/L, and sulphide concentrations increased to 65 mg/L (Fig. 6a).
Aiming further improve sulphate reduction, the bioreactor flow was decreased to 1.7 mL/h (HRT of 9.8 days) on day 246. This improved sulphate reduction, and sulphate and sulphide concentrations stabilized at 812 ± 23 mg/L and 129 ± 9 mg/L, respectively, resulting in a sulphate removal efficiency of 62 ± 1% between days 309 and 345. On day 345, the bioreactor flow was decreased again, to 1.3 mL/h (HRT of 12.8 days), and the SRB activity improved to an even higher efficiency. The sulphate removal rate between days 357 and 380 (when the experiment ended) was 75 ± 2%, with sulphate and sulphide concentrations of 456 ± 41 mg/L and 246 ± 9 mg/L, respectively. This corresponds to 1.31 mol of sulphate removed per day per m3 of support bed media.
The comparison of different studies performed with different, or even the same, organic substrates is difficult because of different durations and/or different ratios of AMD and organics in the reactive mixture used in each study. Nevertheless, it is known that anaerobic degradation of complex organics to simpler molecules by microorganisms influences the rate at which nutrients become available to SRB; that is, complex molecules require longer HRTs than the simple compounds that are directly usable by SRB (Neculita et al. 2007; Sheoran et al. 2010). In a review paper, Hao et al. (2014) published a table summarizing sulphate reduction rates, HRTs, and benefits/drawbacks of using different electron donors, in which they reported a wide range of HRTs (1–480 h). The HRT of 12.8 days (≈307 h) optimized at the end of the current experiment (see Fig. 5) is in the range of the HRTs reported in that review. For example, the HRT reported for benzene and benzoate was 264 h and for cheese whey and animal manure was 192 h and 216 h, respectively.
Metal and phenol removal efficiencies: Focusing on the period with high SRB activity after the first HRT increase on day 246 (with limestone added to the bioreactor packed bed; see Fig. 5), the following was observed (Fig. 6b, c): (1) Fe, Al, and Cu, which were largely removed in the neutralization step, remained at similar concentrations after passing through the bioreactor; (2) Zn, which was still present at relatively high concentrations in the nsAMD exiting the neutralization tank, was largely removed in the bioreactor (to 0.3 mg/L, 98% average removal); (3) Mn, the other metal with high inflow concentration in the nsAMD, was not as efficiently removed (70% removal), but did decrease (to 3.1 mg/L; Table 5).
The removal of Zn in the bioreactor can be attributed to the formation of ZnS precipitates. Lewis (2010) has published a graph showing the pH dependence of metal sulphide solubilities. At neutral pH, such as in the bioreactor under optimized conditions (pH = 7.0 ± 0.3 from day 246 to end), the theoretical solubility of zinc sulphide is ≈ 0.0001 mg/L Zn (Lewis 2010). In fact, batch studies (Pinto da Costa et al. 2012) and flow-through tests (Vitor et al. 2015) previously carried out using lactate and ethanol as carbon sources/electron donors have shown that sulphide generated by SRB is able to remove Zn, yielding nanosized ZnS precipitates. On the other hand, the solubility of manganese sulphide at neutral pH is ≈ 10 mg/L Mn (Lewis 2010), which explains its incomplete removal in the bioreactor. Another possible Mn removal mechanism is ion exchange with Ca in the limestone, causing the formation of MnCO3 and the release of Ca2+.
The concentration of total phenols in the bioreactor outlet during the experiment was not influenced by sulphate reduction. The changes in total phenol concentrations over time was mainly due to dilution (see Figs. 5, 6). In addition, some removal of total phenols was observed, but to a lesser extent than in the batch experiments (80–90% removal). Under the most favourable conditions for sulphate reduction in the bioreactor (20% OMWW and 80% nAMD), total phenol concentrations in the outlet (55 ± 4 mg/L) decreased by ≈50% (Table 5).
Potential Utility of the System
Directive 2000/60/EC, amended by water policy directives 2008/105/EC and 2013/39/EU of the European Parliament and of the Council, describes environmental quality standards (EQS) for pollutants classified as priority substances at community level and leaves it to the discretion of the member states to establish (if necessary) rules for other pollutants at the national level. The major metals present in the AMD studied in this work are not included in these directives. However, in Annex II to Directive 2010/75/EU concerning integrated pollution prevention and control, “Metals and their compounds” are in the “List of polluting substances relevant for fixing emission limit values,” and must be considered by member countries. For example, the limits for metals in this work (as well as for sulphate, sulphide, and total phenols) in wastewater discharges and waters used for irrigation in Portugal, are set in Decree-law no. 236/98 (Table 6).
On the other hand, although phenols are a major difficulty in OMWW detoxification, at the same time they can be a source of valuable products, such as phenolic compounds with antioxidant activity (Obied et al. 2005). Thus, the future management strategy of OMWW treatment should be combining detoxification with production of valuable phenolic by-products. However, at present, such solutions for OMWW treatment require the use of sophisticated technical processes that most of the small, geographically-scattered olive mills cannot afford.
The system tested in this work combined a passive chemical step comprised of a limestone-filled tank with a biological step that is basically a SRB-enriched compost bioreactor that is permanently (or routinely) fed with OMWW as a source of carbon and electron donors instead of having the organic substrates added at the time of construction. This type of semi-passive treatment system is relatively easy and inexpensive to construct and maintain. Moreover, as an added benefit, it may also produce valuable by-products: bioremediated OMWW is an excellent fertilizer (Cereti et al. 2004; Mekki et al. 2006) and can serve as a substrate for nitrogen-fixing bacteria or for polymer production (Balis et al. 1996).
The tested system produces an effluent in compliance with the emission limit values (ELVs) of wastewaters discharges for all studied compounds except Mn, S2−, and total phenols. For irrigation waters, the effluent does comply with the maximum recommended values (MRVs) for all compounds except Mn and possibly Cu (the detection limit of 0.4 mg/L is higher than the MRV of 0.2 mg/L) and complies with the maximum admissible values (MAVs) for all compounds. Therefore, if the objective of such a process for the co-treatment of AMD and OMWW is to discharge the treated effluent in surface waters, further research is needed to optimize the removal of Mn, phenols, and S2− (or the evaporating H2S—vapor pressure ≈ 13 mm Hg at 21 °C). If the objective is to produce water for irrigation (phenol concentration is not regulated for irrigation waters), this depends on the tolerance of the target plants to the final phenol content, and other potential effects have to be considered: that of OMWW phenol degradation on irrigated soils vs. transport of OMWW phenols to waterbodies and their effects on those ecosystems. In any case, since harm to ecological systems and public heath by H2S has driven the control and abatement of this gas at its source, it will be important to evaluate and include systems for H2S removal (e.g. biofiltration (Vikrant et al. 2018) or adsorption (Bamdad et al. 2018)). For example, the use of iron oxide rich materials (steel wool, iron filings, scrap iron, etc.), one of the oldest methods still in practice, is a simple and low-cost way to remove H2S from gas streams by forming Fe2S3 (e.g. Choudhury et al. 2019). Future work could include testing the process in a one-step system comprised of a SRB-based bioreactor filled with limestone, or limestone mixed with another material, which would generate less metal oxide sludge and higher amounts of more stable metal sulphide precipitates, as well as an effluent with a lower sulphide concentration.
Bacterial Communities
Although DNA extraction, library preparation, and sequencing were successful for the four samples, the concentration of purified DNA and the final number of 16S rRNA reads obtained was relatively low for sample AR (batch test without marble powder; Table 7). The most likely explanation for the low number of reads was less biological mass in that sample compared with the other three, probably due to slower bacterial growth caused by the initial acidity of the medium in that batch test. However, because the study was focused on the most common taxonomic groups (> 10% relative abundance) and not on the rarer taxa, the lower number of reads are less of a concern. The 20 most abundant bacteria estimated by the 16S rRNA reads obtained for the four samples are shown in Table 8 and in circular graphs available in the Online Resource (Fig. OR8).
As expected, the primary communities in the enriched seeding culture used as an inoculum was composed of SRB able to oxidize lactate, the carbon source and electron donor present in the growth medium (Postgate B), and consists of species from two genera: Desulfomicrobium (38.8%) and Desulfovibrio (22.9%), with the other detected OTUs having percentages < 4%. Desulfomicrobium spp. use simple organic molecules as electron donors for sulphate respiration (such as hydrogen, formate, ethanol, pyruvate, and lactate) and can also be fermentative using other simple organic compounds (such as fumarate, malate, and pyruvate), although they cannot ferment carbohydrates (Genthner and Devereux 2015). Desulfovibrio spp. can also conduct respiratory metabolism with sulphate as the terminal electron acceptor in the oxidation of simple organic compounds or a fermentative metabolism (Kuever et al. 2015).
The results from samples collected at the end of both batch experiments with 75% (v/v) AMD, 20% (v/v) OMWW, and 5% (v/v) of an SRB-enriched inoculum, one without marble powder and the other with 10% (w/v) marble powder, reveal different bacterial communities. The main difference is that in the culture without marble powder, SRB bacteria from the genus Desulfovibrio (59.5% relative abundancy) are dominant, whereas in the culture with marble powder, this genus represents a smaller proportion (15% relative abundancy) of the community, and bacteria from the genus Sulfurospirillum are dominant (70% relative abundancy). This may seem to contradict the fact that sulphate reduction was less efficient in the culture without marble powder than in the culture with it, yet most probably is a consequence of that fact. In the culture without marble powder, the acidity of the medium at the beginning may have prevented rapid proliferation of bacteria from Desulfovibrio genus, but after a week, the pH became neutral and promoted bacterial growth, as indicated by the gradual decrease in sulphate concentration during that period. In contrast, in the culture with marble powder, the proliferation of Desulfovibrio bacteria was faster because sulphate was almost completely reduced to sulphide after just 2 weeks, during which time this genus likely became the dominant population. After that time, due to limited sulphate, the bacterial community likely switched to Sulfurospirillum as the dominant genus and Desulfovibrio became the second most common genus. Typical electron acceptors for Sulfurospirillum species are toxic constituents such as arsenate, selenite, nitrate, and sulphur compounds (Goris and Diekert 2016). In the final part of the experiment with marble powder, some of the sulphide previously released by the SRB was likely converted to elemental sulphur, which could then be used by Sulfurospirillum bacteria. In previous work, elemental sulphur was one of the compounds detected in precipitates collected from an SRB-enriched bioreactor used to test wine wastes as a carbon source to treat AMD (Costa et al. 2009).
In addition to the groups of bacteria referred above, the Deferribacteraceae family was also prominent in the batch test without marble powder (10.9% relative abundancy). These bacteria perform anaerobic respiration using Fe, Mn, or nitrate as electron acceptors (Huber and Stetter 2001). Therefore, the relatively high concentrations of Mn and nitrate at the beginning of batch test without marble powder could have favoured their proliferation (see Tables 1 and 2). The neutralization from pH 2.48 in the raw wAMD to pH 6.32 in the nwAMD used in this test caused a decrease on the dissolved Fe concentration, as described above. However, Mn concentrations did not decrease much, as confirmed in the later experiment with the continuous system (Table 4). On the other hand, the high concentration of total nitrogen measured in the OMWW tested (Table 2) suggests the presence of nitrate. Moreover, OMWW can prevent nitrate–N loss in a lightly sulphuric-acid diluted medium at room temperature, retaining up to 80% of nitrate–N (Aguilar 2010). The elevated N concentrations likely favoured the growth of Deferribacteraceae bacteria in the test without marble powder. When OMWW was added to soils, it stimulated the growth of bacterial groups involved in N cycling (Karpouzas et al. 2010).
The most prominent bacteria in the sample collected from the middle of the continuous bioreactor were from the Sphingomonadaceae and Prevotellaceae families (with 24.28 and 22.08% relative abundances, respectively) and from the genus Acetobacter (20.3% relative abundancy). Curiously, unlike in the batch tests, the bacteria from Desulfovibrio and Sulfurospirillum genera, despite being among the 20 most abundant groups in this sample, had comparatively low relative abundances (0.8% and 0.2%, respectively). This is likely because in the batch tests the OMWW was added only in the beginning, while in the continuous bioreactor it was always being added. Therefore, in the batch tests, there must have been an evolution of the bacterial populations over time, determined by their ability to adapt to the successively changing conditions, whereas in the continuous bioreactor, there must have been a distribution of different populations, creating stratified layers with different bacterial communities.
The characteristics of the three most abundant groups in the sample from the continuous bioreactor suggests that in the middle part of the bioreactor (the sample collection point), bacteria with an important role in the degradation of the most complex carbon sources in OMWW predominate. In the batch tests, these bacteria likely proliferated initially, creating optimal conditions for the growth of those that were detected in greater abundance at the end of the experiment.
Several species of the Sphingomonadaceae family, which are usually isolated from soils, water habitats, activated sludge, or plants’ phyllosphere or rhizosphere, can degrade natural or anthropogenic recalcitrant (poly)aromatic compounds; therefore, they are useful in bioremediation applications (Glaeser and Kämpfer 2014). There is other evidence that bacteria from this family are important for OMWW bioremediation. For example, it was found that in the three OMWWs produced from three olive tree varieties, at least 15% of the OTUs were common, and the most abundant were from the Moraxellaceae, Pseudomonadaceae, Sphingomonadaceae, and Microbacteriaceae families (Tsiamis et al. 2012).
Prevotellaceae are among the most common culturable bacteria from the rumen and hind gut of cattle and sheep, where they help in the breakdown of protein and carbohydrate derived from plant material (Rosenberg 2014). Although this family is not normally mentioned as being important for the bioremediation of recalcitrant pollutants, this and other published work support that idea. For example, in a study to assess the potential of rumen microbiome to detoxify hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX), an explosive that causes severe environmental contamination, it was concluded that members of the genus Prevotella were linked to its detoxification (Perumbakkam and Craig 2012). A study based on 16S rRNA gene libraries constructed using PCR primers for β-Proteobacteria to identify bacterial diversity in OMWW revealed that bacterial diversity in O. europaea var. mastoidis-generated OMWW consisted mainly of members of Acetobacteriaceae, Prevotellaceae, and Lactobacillaceae families (Kavroulakis and Ntougias 2011).
Acetobacter spp. are aerophile/microaerophile bacteria that can oxidize ethanol to acetic acid/acetate and ultimately to water and CO2 (Saichana et al. 2015; Sengun and Karabiyikli 2011). These bacteria are used in vinegar manufacturing because they do not attack acetate if ethanol is present (Jucker and Ettlinger 1985). The proliferation of Acetobacter strains indicates the presence of oxygen at the sampling point, which likely remained in low concentrations from the nAMD and OMWW. However, it is not known if the Acetobacter detected in the bioreactor resulted from the ethanol produced by fermentation of OMWW’s sugars or from the acetate produced by the SRB, or both. SRB can use several organic compounds (such as carboxylic acids or alcohols) as energy sources, if they are incompletely oxidized with acetate as a by-product, or by their complete oxidation leading to the final production of water and carbon dioxide (LiamLeam and Annachatre 2007; Parshina et al. 2010; Postgate 1984). The Desulfovibrio strains, the most common SRB in this process, are particularly known for oxidizing carbon compounds used as energy sources to acetate (Postgate 1984).
Conclusions
Using OMWW as a supplemental source of carbon and electron donors for the bioremediation of AMD with SRB-enriched bioreactors requires a neutralizing agent (such as marble powder or crushed limestone) to create optimal pH conditions for sulphate reduction. Six groups of bacteria were identified as having a fundamental role in the bioremediation process: the genera Desulfovibrio, Sulfurospirillum, and Acetobacter, and the families Sphingomonadaceae, Prevotellaceae, and Deferribacteraceae.
In such a process, sulphate and the metals Al, Fe, Cu, Zn, and Mn (common pollutants in AMD) can be reduced to concentrations below Portuguese MAVs for irrigation waters, and all except Mn were reduced to concentrations below the ELVs for wastewater discharges. Regarding phenols (the main pollutant in OMWW), although total phenols were reduced by 80–90% in batch tests and by ≈ 50% in the continuous-flow test, using a mix of 20% OMWW and 80% AMD, their final concentrations were still above the limit for wastewater discharges. Nevertheless, this work represents the starting point for development of a new bioremediation system for co-treatment of AMD and OMWW. Moreover, the work raises the possibility that phenol degradation in OMWW can be catalysed by chemical species present in AMD, encouraging further research aimed at developing processes using AMD for the treatment of waters contaminated with recalcitrant organics.
References
Aguilar MJ (2010) Fixation of ammonium-N and nitrate-N with olive oil mill wastewaters. Environ Technol 31:395–398. https://doi.org/10.1080/09593330903501836
Albertsen M, Karst SM, Ziegler AS, Kirkegaard RH, Nielsen PH (2015) Back to basics—the influence of DNA extraction and primer choice on phylogenetic analysis of activated sludge communities. PLoS ONE 10:e0132783. https://doi.org/10.1371/journal.pone.0132783
Alvarenga P, Palma P, de Varennes A, Cunha-queda AC (2012) A contribution towards the risk assessment of soils from the São Domingos Mine (Portugal): chemical, microbial and ecotoxicological indicators. Environ Pollut 161:50–56. https://doi.org/10.1016/j.envpol.2011.09.044
Álvarez-Valero AM, Pérez-López R, Matos J, Capitán MA, Nieto JM, Sáez R, Delgado J, Caraballo M (2008) Potential environmental impact at São Domingos mining district (Iberian pyrite belt, SW Iberian Peninsula): evidence from a chemical and mineralogical characterization. Environ Geol 55:1797–1809. https://doi.org/10.1007/s00254-007-1131-x
Amaral C (2009) Caracterização Físico-Química e Microbiológica de Águas Residuais de Lagares de Azeite—Selecção de Leveduras para Aplicação ao Tratamento de Águas Ruças. PhD thesis, University of Trás-os-Montes and Alto Douro. https://hdl.handle.net/10348/2112
Amaral C, Lucas MS, Coutinho J, do Crespí AL, Rosário Anjos M, Pais C (2008) Microbiological and physicochemical characterization of olive mil wastewaters from a continuous olive mill in Northeastern Portugal. Bioresour Technol 99:7215–7223. https://doi.org/10.1016/j.biortech.2007.12.058
Azbar N, Bayram A, Filibeli A, Muezzinoglu A, Sengul F, Ozer A (2004) A review of wastes management options in olive oil production. Crit Rev Environ Sci Technol 34:209–247. https://doi.org/10.1080/10643380490279932
Balis C, Chatzipavlidis J, Flouri F (1996) Olive mill waste as a substitute for nitrogen fixation. Int Biodeter Biodegr 38:169–178. https://doi.org/10.1016/S0964-8305(96)00047-9
Bamdad H, Hawboldt K, MacQuarrie S (2018) A review on common adsorbents for acid gases removal: focus on biochar. Renew Sust Energ Rev 81(2):1705–1720. https://doi.org/10.1016/j.rser.2017.05.261
Barros RJ, Jesus C, Martins M, Costa MC (2009) Marble stone processing powder residue as chemical adjuvant for the biologic treatment of acid mine drainage. Process Biochem 44:477–480. https://doi.org/10.1016/j.procbio.2008.12.013
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Boonstra J, van Lier R, Janssen G, Dijkman H, Buisman CJN (1999) Biological treatment of acid mine drainage. In: Amils R, Ballester A (eds) Biohydrometallurgy and the environment toward the mining of the 21st century, vol 9B. Elsevier, Amsterdam, pp 559–567
Brown DE, Groves GR, Miller JDA (1973) pH and Eh control of cultures of sulphate-reducing bacteria. J Appl Chem Biotech 23:141–149. https://doi.org/10.1002/jctb.5020230210
Cao J, Zhang G, Mao Z, Li Y, Fang F, Chao Y (2012) Influence of electron donors on the growth and activity of sulphate-reducing bacteria. Int J Miner Process 106–109:58–64. https://doi.org/10.1016/j.minpro.2012.02.005
Capasso R, Evidente A, Schivo L, Orru G, Marcialis MA, Cristinzio G (1995) Antibacterial polyphenols from olive oil mill waste waters. J Appl Microbiol 79:393–398. https://doi.org/10.1111/j.1365-2672.1995.tb03153.x
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Je Z, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. https://doi.org/10.1038/nmeth.f.303
Caporaso JG, Lauber CL, Walters W, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, GormLey N, Gilbert JA, Smith G, Knight R (2012) Ultra-high throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624. https://doi.org/10.1038/ismej.2012.8
Carlier JD, Alexandre LM, Luís AT, Costa MC (2019) Potential of industrial by-products and wastes from the Iberian Peninsula as carbon sources for sulphate-reducing bacteria. Int J Environ Sci Technol 16:4719–4738. https://doi.org/10.1007/s13762-018-02197-z
Cereti CF, Rossini F, Federici F, Quaratino D, Vassilev N, Fenice M (2004) Reuse of microbially treated olive mill wastewater as fertilizer for wheat (Triticum durum Desf.). Bioresour Technol 91:135–140. https://doi.org/10.1016/S0960-8524(03)00181-0
Chamkh F, Sproer C, Lemos P, Besson S, El Asli A, Bennisse R, Qatibi A (2009) Desulfovibrio marrakechensis sp. nov., a 1,4-tyrosol-oxidizing, sulfate-reducing bacterium isolated from olive mill wastewater. Int J Syst Evol Micr 59:936–942. https://doi.org/10.1099/ijs.0.003822-0
Choudhury A, Shelford T, Felton G, Gooch C, Lansing S (2019) Evaluation of hydrogen sulfide scrubbing systems for anaerobic digesters on two US dairy farms. Energies 12:4605–4617. https://doi.org/10.3390/en12244605
Cohen RRH (2006) Use of microbes for cost reduction of metal removal from metals and mining industry waste streams. J Clean Prod 14:1146–1157. https://doi.org/10.1016/j.jclepro.2004.10.009
Costa MC, Duarte JC (2005) Bioremediation of acid mine drainage using acidic soil and organic wastes for promoting sulphate-reducing bacteria activity on a column reactor. Water Air Soil Poll 165:325–345. https://doi.org/10.1007/s11270-005-6914-7
Costa MC, Martins M, Jesus C, Duarte JC (2008) Treatment of acid mine drainage by sulphate-reducing bacteria using low cost matrices. Water Air Soil Poll 189:149–162. https://doi.org/10.1007/s11270-007-9563-1
Costa MC, Santos ES, Barros RJ, Pires C, Martins M (2009) Wine wastes as carbon source for biological treatment of acid mine drainage. Chemosphere 75:831–836. https://doi.org/10.1016/j.chemosphere.2008.12.062
de Souza e Silva PT, da Silva deBarros Neto VLB, Simonnot M-O (2009) Potassium permanganate oxidation of phenanthrene and pyrene in contaminated soils. J Hazard Mater 168:1269–1273. https://doi.org/10.1016/j.jhazmat.2009.03.007
Decree-Law (2020) No. 236/98. Diário da República n.º 176/1998, Série I-A de 1998–08–01, p. 3676–3722. https://data.dre.pt/eli/dec-lei/236/1998/08/01/p/dre/pt/htmL. Accessed 20 July 2020
Dias-Sardinha I, Craveiro D, Milheiras S (2013) A sustainability framework for redevelopment of rural brownfields: stakeholder participation at São Domingos mine, Portugal. J Clean Prod 57:200–208. https://doi.org/10.1016/j.jclepro.2013.05.042
Diem D, Stumm W (1984) Is dissolved Mn 2+ being oxidized by O2 in absence of Mn-bacteria or surface catalysts? Geochim Cosmochim Acta 48:1571–1573. https://doi.org/10.1016/0016-7037(84)90413-7
Directive (2000) 2000/60/EC. OJ L 327, 22.12.2000, p. 1–73. https://data.europa.eu/eli/dir/2000/60/oj. Accessed 20 July 2020
Directive (2008) 2008/105/EC. OJ L 348, 24.12.2008, p. 84–97. https://data.europa.eu/eli/dir/2008/105/oj. Accessed 20 July 2020
Directive (2010) 2010/75/EU. OJ L 334, 17.12.2010, p. 17–119. https://data.europa.eu/eli/dir/2010/75/oj. Accessed 20 July 2020
Directive (2013) 2013/39/EU. OJ L 226, 24.8.2013, p. 1–17. https://data.europa.eu/eli/dir/2013/39/oj. Accessed 20 July 2020
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998. https://doi.org/10.1038/nmeth.2604
Egiebor NO, Oni B (2007) Acid rock drainage formation and treatment: a review. Asia-Pac J Chem Eng 2:47–62. https://doi.org/10.1002/apj.57
Elhag M, Bahrawi JA, Galal HK, Galal HK, Aldhebiani A, Al-Ghamdi AAM (2017) Stream network pollution by olive oil wastewater risk assessment in Crete, Greece. Environ Earth Sci 76:278–289. https://doi.org/10.1007/s12665-017-6592-y
Fang G-D, Dionysiou D, Al-Abed SR, Zhou D-M (2013) Superoxide radical driving the activation of persulfate by magnetite nanoparticles: implications for the degradation of PCBs. Appl Catal B-Environ 129:325–332. https://doi.org/10.1016/j.apcatb.2012.09.042
FAOSTAT (2014) Production—Crops processed. https://www.fao.org/faostat/en/#data/QD
Gazea B, Adam K, Kontopoulos A (1996) A review of passive systems for the treatment of acid mine drainage. Miner Eng 9:23–42. https://doi.org/10.1016/0892-6875(95)00129-8
Genthner BRS, Devereux R (2015) Desulfomicrobium. In: Whitman WB (ed) Bergey's manual of systematics of archaea and bacteria. Wiley, Hoboken. https://doi.org/10.1002/9781118960608.gbm01032(In association with Bergey's Manual Trust)
Girts MA, Kleinmann LP, Erickson PM (1987) Performance data on Typha and Sphagnum wetlands constructed to treat coal mine drainage. In: Proc, 8th Annual surface mine drainage task force symp, Morgantown, WV
Glaeser SP, Kämpfer P (2014) The family Sphingomonadaceae. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (eds) The prokaryotes. Springer, Berlin. https://doi.org/10.1007/978-3-642-30197-1_302
Goris T, Diekert G (2016) The genus Sulfurospirillum. In: Adrian L, Löffler F (eds) Organohalide-respiring bacteria. Springer, Berlin. https://doi.org/10.1007/978-3-662-49875-0_10
Hao TW, Xiang PY, Mackey HR, Chi K, Lu H, Chui HK, van Loosdrecht MC, Chen GH (2014) A review of biological sulfate conversions in wastewater treatment. Water Res 65:1–21. https://doi.org/10.1016/j.watres.2014.06.043
Huber H, Stetter KO (2001) Family I. Deferribacteraceae fam. nov. In: Boone DR, Castenholz RW, Garrity GM (eds) Bergey's manual of systematic bacteriology, vol 1: the archaea and the deeply branching and phototrophic bacteria, 2nd edn. Springer, NYC, pp 465–466. https://doi.org/10.1007/978-0-387-21609-6
Jeguirim M, Dutournié P, Zorpas AA, Limousy L (2017) Olive mill wastewater: from a pollutant to green fuels, agricultural water source and bio-fertilizer—part 1 the drying kinetics. Energies 10:1423. https://doi.org/10.3390/en10091423
Johnson DB (2003) Chemical and microbiological characteristics of mineral spoils and drainage waters at abandoned coal and metal mines. Water Air Soil Poll 3:47–66. https://doi.org/10.1023/A:1023977617473
Johnson DB, Hallberg KB (2005) Acid mine drainage remediation options: a review. Sci Total Environ 338:3–14. https://doi.org/10.1016/j.scitotenv.2004.09.002
Jucker W, Ettlinger L (1985) The inhibition of acetate oxidation by ethanol in Acetobacter aceti. Arch Microbiol 143:283–289. https://doi.org/10.1007/BF00411251
Karpouzas DG, Ntougias S, Iskidou E, Rousidou C, Papadopoulou KK, Zervakis GI, Ehaliotis C (2010) Olive mill wastewater affects the structure of soil bacterial communities. Appl Soil Ecol 45:101–111. https://doi.org/10.1016/j.apsoil.2010.03.002
Katsoyiannis IA, Zouboulis AI (2004) Biological treatment of Mn(II) and Fe(II) containing groundwater: kinetic considerations and product characterization. Water Res 38:1922–1932. https://doi.org/10.1016/j.watres.2004.01.014
Kavroulakis N, Ntougias S (2011) Bacterial and β-proteobacterial diversity in Olea europaea var. mastoidis- and O. europaea var. koroneiki-generated olive mill wastewaters: influence of cultivation and harvesting practice on bacterial community structure. World J Microb Biot 27:57–66. https://doi.org/10.1007/s11274-010-0426-3
Kefeni KK, Msagati TAM, Mamba BB (2017) Acid mine drainage: prevention, treatment options, and resource recovery: a review. J Clean Prod 151:475–493. https://doi.org/10.1016/j.jclepro.2017.03.082
Khan A, Liao Z, Liu Y, Jawad A, Ifthikar J, Chen Z (2017) Synergistic degradation of phenols using peroxymonosulfateactivated by CuO-Co3O4@MnO2 nanocatalyst. J Hazard Mater 329:262–271. https://doi.org/10.1016/j.jhazmat.2017.01.029
Kleinmann RLP, Hedin RS (1993) Treat mine water using passive methods. Pollut Eng 25:20–22
Kleinmann RLP, Tiernan TO, Solch JG, Harris RL (1983) A low-cost, low-maintenance treatment system for acid mine drainage using Sphagnum moss and limestone. In: Proc, 1983 Symp. on Surface Mining, Hydrology, Sedimentology and Reclamation, Univ of Kentucky, Lexington
Kleinmann RLP, Hedin RS, Nairn RW (1998) Treatment of mine drainage by anoxic limestone drains and constructed wetlands. In: Geller A, Klapper H, Salomons W (eds) Acidic mining lakes: acid mine drainage, limnology and reclamation. Springer, Berlin, pp 303–319
Koschorreck M (2008) Microbial sulphate reduction at a low pH. FEMS Microbiol Ecol 64:329–342. https://doi.org/10.1111/j.1574-6941.2008.00482.x
Koutsos TM, Chatzistathis T, Balampekou EI (2018) A new framework proposal, towards a common EU agricultural policy, with the best sustainable practices for the re-use of olive mill wastewater. Sci Total Environ 622–623:942–953. https://doi.org/10.1016/j.scitotenv.2017.12.073
Kuever J, Rainey FA, Widdel F (2015) Desulfovibrio. In: Whitman WB, Rainey F, Kämpfer P, Trujillo M, Chun J, DeVos P, Hedlund B, Dedysh S (eds) Bergey’s manual of systematics of archaea and bacteria. Wiley, Hoboken, pp 1–17. https://doi.org/10.1002/9781118960608.gbm01035(In association with Bergey's Manual Trust)
Levén L, Nyberg K, Schnürer A (2012) Conversion of phenols during anaerobic digestion of organic solid waste—a review of important microorganisms and impact of temperature. J Environ Manage 95:S99–S103. https://doi.org/10.1016/j.jenvman.2010.10.021
Lewis AE (2010) Review of metal sulphide precipitation. Hydrometallurgy 104:222–234. https://doi.org/10.1016/j.hydromet.2010.06.010
LiamLeam W, Annachatre AP (2007) Electron donors for biological sulfate reduction. Biotechnol Adv 25:452–463. https://doi.org/10.1016/j.biotechadv.2007.05.002
Lovett RJ (1992) Removal of manganese from acid mine drainage. Proc, 1992 West Virginia Surface Mine Drainage Task Force Symp, Morgantown, pp 97–102. https://wvmdtaskforce.files.wordpress.com/2015/12/92-lovett.pdf
Luan F, Santelli CM, Hausel CM, Burgos WB (2012) Defining manganese (II) removal processes in passive coal mine drainage treatment systems through laboratory incubation experiments. Appl Geochem 27:1567–1578. https://doi.org/10.1016/j.apgeochem.2012.03.010
Magoc T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. https://doi.org/10.1093/bioinformatics/btr507
Maree JP, Hill GE (1987) An integrated process for biological treatment of sulfate-containing industrial effluents. J Water Poll Con F 59:1069–1074. https://www.jstor.org/stable/25043436
Martins M, Faleiro ML, Barros RJ, Veríssimo AR, Barreiros MA, Costa MC (2009) Characterization and activity studies of highly heavy metal resistant sulphate-reducing bacteria to be used in acid mine drainage decontamination. J Hazard Mater 166:706–713. https://doi.org/10.1016/j.jhazmat.2008.11.088
Mekki A, Dhouib A, Aloui F, Sayadi S (2006) Olive wastewater as an ecological fertilizer. Agron Sustain Dev 26:61–67. https://doi.org/10.1051/agro:2005061
Mezyk SP, Rickman KA, McKay G, Hirsch CM, He X, Dionysiou DD (2011) Remediation of chemically-contaminated waters using sulfate radical reactions: kinetic studies. In: Tratnyek PG, Grundl TJ, Haderlein SB (eds) Aquatic redox chemistry. American Chemical Society, Washington, pp 247–263. https://doi.org/10.1021/bk-2011-1071.ch012
Nassar NN, Arar LA, Marei NN, Abu Ghanim MM, Dwekat MS, Sawalha SH (2014) Treatment of olive mill-based wastewater by means of magnetic nanoparticles: decolourization, dephenolization and COD removal. Environ Nanotechnol Monit Manage 1–2:14–23. https://doi.org/10.1016/j.enmm.2014.09.001
Neculita CM, Zagury GJ, Bussiere B (2007) Passive treatment of acid mine drainage in bioreactors using sulphate-reducing bacteria: critical review and research needs. J Environ Qual 36:1–16. https://doi.org/10.2134/jeq2006.0066
Niaounakis M, Halvadakis CP (2006) Olive-mill waste management—literature review and patent survey. In: Niaounakis M, Halvadakis CP (eds) Waste management series, 5th edn. Elsevier, Amsterdam. https://doi.org/10.1016/S1478-7482(13)60004-4
O’Flaherty V, Mahony T, Kennedy O, Colleran E (1998) Effect of pH on growth kinetics and sulphide toxicity thresholds of a range of methanogenic, syntrophic and sulphate-reducing bacteria. Process Biochem 33:555–569. https://doi.org/10.1016/S0032-9592(98)00018-1
Obied HK, Llen MS, Bedgood DR, Prenzler PD, Robards K, Stockmann R (2005) Bioactivity and analysis of biophenols recovered from olive mill waste. J Agric Food Chem 53:823–837. https://doi.org/10.1021/jf048569x
Ochando-Pulido JM, Hodaifa G, Víctor-Ortega MD, Martínez-Ferez A (2013) A novel photocatalyst with ferromagnetic core used for the treatment of olive oil mill effluents from two-phase production process. Sci World J. https://doi.org/10.1155/2013/196470
Parshina SN, Sipma J, Henstra AM, Stams AJM (2010) Carbon monoxide as an electron donor for the biological reduction of sulphate. Int J Microbiol. https://doi.org/10.1155/2010/319527
Pereira R, Ribeiro R, Gonçalves F (2004) Plan for an integrated human and environmental risk assessment in the S. Domingos Mine area (Portugal). Hum Ecol Risk Assess An Int J 10:543–578. https://doi.org/10.1080/10807030490452197
Perumbakkam S, Craig AM (2012) Biochemical and microbial analysis of ovine rumen fluid incubated with 1,3,5-trinitro-1,3,5-triazacyclohexane (RDX). Curr Microbiol 65:195–201. https://doi.org/10.1007/s00284-012-0144-1
Pinto da Costa J, Girão AV, Lourenço JP, Monteiro OC, Trindade T, Costa MC (2012) Synthesis of nanocrystalline ZnS using biologically generated sulfide. Hydrometallurgy 117–118:57–63. https://doi.org/10.1016/j.hydromet.2012.02.005
Postgate JR (1984) The sulphate-reducing bacteria, 2nd edn. Cambridge Univ Press, UK
Puigdomenech I (2015) Hydra/medusa chemical equilibrium database and plotting software (database update: 01–01–2015; Hydra: 32 bit version 18 Aug. 2009; Medusa: 32 bit version 16 Dec. 2010). KTH Royal Institute of Technology, Stockolm
Robbins EI, Brant DL, Ziemkiewicz PF (1999) Microbial, algal and fungal strategies for manganese oxidation at a Shade Township coal mine, Somerset County, Pennsylvania. Proc Am Soc Min Reclam 1999:634–640. https://doi.org/10.21000/JASMR99010634
Rosenberg E (2014) The family Prevotellaceae. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (eds) The prokaryotes. Springer, Berlin. https://doi.org/10.1007/978-3-642-38954-2_131
Saichana N, Matsushita K, Adachi O, Frébort I, Frebortova J (2015) Acetic acid bacteria: a group of bacteria with versatile biotechnological applications. Biotechnol Adv 33:1260–1271. https://doi.org/10.1016/j.biotechadv.2014.12.001
Sayadi S, Allouche N, Jaoua M, Aloui F (2000) Detrimental effects of high molecular-mass polyphenols on olive mill wastewater biotreatment. Process Biochem 35:725–735. https://doi.org/10.1016/S0032-9592(99)00134-X
Sengun IY, Karabiyikli S (2011) Importance of acetic acid bacteria in food industry. Food Control 22:647–656. https://doi.org/10.1016/j.foodcont.2010.11.008
Seyler J, Figueroa L, Ahmann D, Wildeman TR, Robustelli M (2003) Proc Am Soc Min Reclam. https://doi.org/10.21000/JASMR03011112
Sheoran AS, Sheoran V, Choudhary RP (2010) Bioremediation of acid-rock drainage by sulphate reducing prokaryotes: a review. Miner Eng 23:1073–1100. https://doi.org/10.1016/j.mineng.2010.07.001
Simate GS, Ndlovu S (2014) Acid mine drainage: challenges and opportunities. J Environ Chem Eng 2:1785–1803. https://doi.org/10.1016/j.jece.2014.07.021
Skousen J, Zipper CE, Rose A, Ziemkiewicz PF, Nairn R, McDonald LM, Kleinmann RL (2017) Review of passive systems for acid mine drainage treatment. Mine Water Environ 36:133–153. https://doi.org/10.1007/s10230-016-0417-1
Stumm W, Morgan JJ (1995) Aquatic chemistry: chemical equilibria and rates in natural waters, 3rd edn. Wiley, NYC
Tavares MT, Sousa AJ, Abreu MM (2008) Ordinary kriging and indicator kriging in the cartography of trace elements contamination in São Domingos mining site (Alentejo, Portugal). J Geochem Explor 98:43–56. https://doi.org/10.1016/j.gexplo.2007.10.002
Tsagaraki E, Lazarides HN, Petrotos KB (2007) Olive mill wastewater treatment. In: Oreopoulou V, Russ W (eds) Utilization of by-products and treatment of waste in the food industry. Springer, NYC, pp 133–157
Tsiamis G, Tzagkaraki G, Chamalaki A, Xypteras N, Andersen G, Vayenas D, Bourtziz K (2012) Olive-mill wastewater bacterial communities display a cultivar specific profile. Curr Microbiol 64:197–203. https://doi.org/10.1007/s00284-011-0049-4
URS (2003) Passive and semi-active treatment of acid rock drainage from metal mines—state of the practice. US Army Corps of Engineers, Portland
Valls M, de Lorenzo V (2002) Exploiting the genetic and biochemical capacities of bacteria for the remediation of heavy metal pollution. FEMS Microbiol Rev 26:327–338. https://doi.org/10.1111/j.1574-6976.2002.tb00618.x
Vikrant K, Kailasa SK, Tsang DCW, Lee SS, Kumar P, Giri BS, Singh RS, Kim K-H (2018) Biofiltration of hydrogen sulfide: trends and challenges. J Clean Prod 187:131–147. https://doi.org/10.1016/j.jclepro.2018.03.188
Vitor G, Palma TC, Vieira B, Lourenço JP, Barros RJ, Costa MC (2015) Start-up, adjustment and long-term performance of a two-stage bioremediation process, treating real acid mine drainage, coupled with biosynthesis of ZnS nanoparticles and ZnS/TiO2 nanocomposites. Miner Eng 75:85–93. https://doi.org/10.1016/j.mineng.2014.12.003
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. https://doi.org/10.1128/AEM.00062-07
Wang Y, Zhou L, Duan X, Sun H, Tin EL, Jin W, Wang S (2015) Photochemical degradation of phenol solutions on Co3O4 nanorods with sulfate radicals. Catal Today 258:576–584. https://doi.org/10.1016/j.cattod.2014.12.020
Wang R, Wang S, Tai Y, Tao R, Dai Y, Guo J, Yang Y, Duan S (2017) Biogenic manganese oxides generated by green algae Desmodesmus sp. WR1 to improve bisphenol A removal. J Hazard Mater 339:310–319. https://doi.org/10.1016/j.jhazmat.2017.06.026
Ward DV, Gevers D, Giannoukos G, Earl AM, Methé BA, Sodergren E et al (2012) Evaluation of 16s rDNA-based community profiling for human microbiome research. PLoS ONE 7:e39315. https://doi.org/10.1371/journal.pone.0039315
Willow MA, Cohen RRH (2003) pH, dissolved oxygen, and adsorption effects on metal removal in anaerobic bioreactors. J Environ Qual 32:1212–1221. https://doi.org/10.2134/jeq2003.1212
Zhang B-T, Zhang Y, Teng Y, Fan M (2015) Sulfate radical and its application in decontamination technologies. Crit Rev Env Sci Tec 45:1756–1800. https://doi.org/10.1080/10643389.2014.970681
Acknowledgements
This work was supported by (1) the European Union’s Seventh Framework Programme (FP7/2007-2013) managed by REA-Research Executive Agency (https://ec.europa.eu/research/rea) under grant agreement 619101, through the project BIOMETALDEMO; (2) the Portuguese Foundation for Science and Technology (FCT) through the project UIDB/04326/2020; and (3) national funds from FCT co-financed by the Algarve´s Regional Operational Program (CRESC Algarve 2020) through Portugal 2020 and European Regional Development Fund (FEDER), under the project METALCHEMBIO (no. 29251). The authors also acknowledge the company Águas do Algarve and Engineers António Martins, Joaquim Freire and Patrício Fontinha for supplying the WWTP sludge used to enrich cultures with SRB, as well as the olive mill Lagar Santa Catarina and its owner Mr. Renato Rocha for supplying the OMWW tested as a source of carbon and electron donors.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carlier, J.D., Luís, A.T., Alexandre, L.M. et al. Feasibility of Co-Treating Olive Mill Wastewater and Acid Mine Drainage. Mine Water Environ 39, 859–880 (2020). https://doi.org/10.1007/s10230-020-00719-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-020-00719-1