Abstract
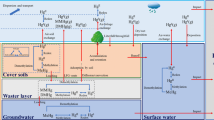
Owing to Minamata Convention on mercury, the final disposal of mercury in environmentally safe manners will be required. Mercury disposal in landfill sites will be one of the feasible options but its environmental risk has been strongly concerned. This study built a model including hydraulic flows of rain infiltration from the top surface, unsaturated percolation in the landfill body, leachate discharge from the collection pipe, mercury transfer including diffusion and sorption, and chemical/biological reactions of mercury species to simulate mercury emissions from a mercury landfill site. Mercury emissions via landfill leachate and landfill gas to the atmosphere were simulated. The model was validated using lab-scale lysimeter experiment data. The model predicted that the major emission pathway of mercury to the environment is landfill leachate, which accounted for 99.8% of the total emissions. 83% of mercury in the leachate was estimated to be inorganic form and the rest 17% was methylmercury. The simulation suggested that mercury emission exceeds the environmental standard after the 16th year. Long-term monitoring of the leachate is necessary even when no mercury detection continues for 16 years. The model proposed that rainwater infiltration control on the top surface is very effective to reduce mercury emissions.








Similar content being viewed by others
References
UN Environment (2019) Global mercury assessment 2018, UN Environment Programme, Chemicals and Health Branch Geneva, Switzerland, ISBN: 978-92-807-3744-8
Lee KJ, Lee TG (2012) A review of international trends in mercury management and available options for permanent or long-term mercury storage. J Hazard Mater 241:1–13
Mukherjee AB, Zevenhoven R, Brodersen J, Hylander LD, Bhattacharya P (2004) Mercury in waste in the European Union: sources, disposal methods and risks. Resour Conserv Recy 42(2):155–182
Chai XL, Hao YX, Li ZG, Zhu W, Zhao WT (2015) The dependence of the methylation of mercury on the landfill stabilization process and implications for the landfill management. Chemosphere 119:828–834
Gworek B, Dmuchowski W, Gozdowski D, Koda E, Osiecka R, Borzyszkowski J (2015) Influence of a municipal waste landfill on the spatial distribution of mercury in the environment. PLoS One 10(7):e0133130
Xi BD, He XS, Wei ZM, Jiang YH, Li D, Pan HW, Liu HL (2012) The composition and mercury complexation characteristics of dissolved organic matter in landfill leachates with different ages. Ecotox Environ Safe 86:227–232
Kim KH, Kim MY, Lee G (2001) The soil–air exchange characteristics of total gaseous mercury from a large-scale municipal landfill area. Atmos Environ 35:3475–3493
Kim KH, Kim MY (2002) Mercury emissions as landfill gas from a large-scale abandoned landfill site in Seoul. Atmos Environ 36:3475–3493
Marti V, Jubany I, Perez C, Rubio X, De Pablo J, Gimenez J (2014) Human health risk assessment of a landfill based on volatile organic compounds emission, immission and soil gas concentration measurements. Appl Geochem 49:218–224
Boening DW (2000) Ecological effects, transport, and fate of mercury: a general review. Chemosphere 40(12):1335–1351
Han YJ, Kim PR, Lee GS, Lee JI, Noh S, Yu SM, Park KS, Seok KS, Kim H, Kim YH (2017) Mercury concentrations in environmental media at a hazardous solid waste landfill site and mercury emissions from the site. Environ Earth Sci 76(10):361
Ullrich SM, Tanton TW, Abdrashitova SA (2001) Mercury in the aquatic environment: a review of factors affecting methylation. Crit Rev Environ Sci Technol 31(3):241–293
Lavoie RA, Jardine TD, Chumchal MM, Kidd KA, Campbell LM (2013) Biomagnification of mercury in aquatic food webs: a worldwide meta-analysis. Environ Sci Technol 47(23):13385–13394
Morel FMM, Kraepiel AML, Amyot M (1998) The chemical cycle and bioaccumulation of mercury. Annu Rev Ecol Syst 29:543–566
Renzoni A, Zino F, Franchi E (1998) Mercury levels along the food chain and risk for exposed populations. Environ Res 77(2):68–72
Fuhrmann M, Melamed D, Kalb PD, Adams JW, Milian LW (2002) Sulfur polymer solidification/stabilization of elemental mercury waste. Waste Manage 22(3):327–333
Piao HS, Bishop PL (2006) Stabilization of mercury-containing wastes using sulfide. Environ Pollut 139(3):498–506
Noyes AA, Whitney WR (1897) The rate of solution of solid substances in their own solutions. J Am Chem Soc 19:930–934
Van Genuchten MT (1980) A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Soc Am J 44:892–898
Drott A, Lambertsson L, Bjorn E, Skyllberg U (2008) Do potential methylation rates reflect accumulated methyl mercury in contaminated sediments? Environ Sci Technol 42(1):153–158
Heyes A, Mason RP, Kim EH, Sunderland E (2006) Mercury methylation in estuaries: insights from using measuring rates using stable mercury isotopes. Marine Chem 102(1–2):134–147
Gray JE, Hines ME, Higueras PL, Adatto I, Lasorsa BK (2004) Mercury speciation and microbial transformations in mine wastes, stream sediments, and surface waters at the Almaden Mining District, Spain. Environ Sci Technol 38(16):4285–4292
Chai XL, Hao YX, Liu GX, Zhao X, Zhao YC (2013) Spectroscopic studies of the effect of aerobic conditions on the chemical characteristics of humic acid in landfill leachate and its implication for the environment. Chemosphere 91(7):1058–1063
Haitzer M, Hoss S, Traunspurger W, Steinberg C (1998) Effects of dissolved organic matter (DOM) on the bioconcentration of organic chemicals in aquatic organisms—a review. Chemosphere 37(7):1335–1362
Japan Meteorological Agency (2020) Precipitation database. Available online: https://www.data.jma.go.jp/gmd/risk/obsdl/index.php. Accessed 31 Oct 2022
Takahashi F (2017) Uncertainty analysis of simulated mercury exposure using a mercury environmental fate model for environmental risk estimate. J Jpn Soc Civ Eng G 73(7):III_307-III_314 (In Japanese)
Koda E, Zakowicz S (1998) Physical and hydraulic properties of the MSW for water balance of the landfill. Environ Geotech 1–4:217–222
White JK, Beaven RP, Powrie W, Knox K (2011) Leachate recirculation in a landfill: some insights obtained from the development of a simple 1-D model. Waste Manage 31(6):1210–1221
Reddy KR, Hettiarachchi H, Parakalla NS, Gangathulasi J, Bogner JE (2009) Geotechnical properties of fresh municipal solid waste at Orchard Hills Landfill, USA. Waste Manage 29(2):952–959
The Chemical Society of Japan (2004) Handbook of chemistry: pure chemistry, 5th edn. Maruzen publishing, Tokyo
National Institutes of Health (NTP) (1992) National Toxicology Program Chemical Repository Database. Research Triangle Park, North Carolina
Buffle J, Zhang Z, Startchev K (2007) Metal flux and dynamic speciation at (Bio)interfaces. Part I: critical evaluation and compilation of physicochemical parameters for complexes with simple ligands and fulvic/humic substances. Environ Sci Technol 41(22):7609–7620
Massman WJ (1999) Molecular diffusivities of Hg vapor in air, O2 and N2 near STP and the kinematic viscosity and thermal diffusivity of air near STP. Atmos Environ 33:453–457
Takahashi F, Takatori T, Shimaoka T (2009) Diffusion coefficient of a protective layer for recovered mercury repository vessel evaluated based on a mercury transportation model. Environ Eng Res Jpn Soc Civ Eng 46:345–354 (In Japanese)
Sano A, Kawase K, Yanase R, Takaoka M, Matsuyama A, Takahashi F, Kato T (2020) Long-term mercury behavior in stabilized/solidified mercury wastes by a simulated landfill experiment using lysimeters. Glob Environ Res (AIRIES) 24(1):35–43
Akagi H, Nishimura H (1991) Speciation of mercury in the environment. In: Suzuki T, Imura N, Clarkson TW (eds) (1st) Advances in Mercury Toxicology. Plenum Press, New York, pp 53–76
Matsuyama A, Eguchi T, Sonoda I, Tada A, Yano S, Tai A, Marumoto K, Tomiyasu T, Akagi H (2011) Mercury speciation in the water of Minamata Bay, Japan. Water Air Soil Poll 218(4):399–412
Hirano H, Takemoto K (2019) Difficulty in inferring microbial community structure based on co-occurrence network approaches. BMC Bioinf 20:329
Ding ZH, Tang QH, Liu CE, Wang WH, Zhuang M, Lin YM (2007) Distribution and ecological effect of mercury in Laogang landfill, Shanghai, China. J Environ Sci 19(2):200–204
Yang J, Takaoka M, Sano A, Matsuyama A, Yanase R (2018) Vertical distribution of total mercury and mercury methylation in a landfill site in Japan. Int J Environ Res Pu 15(6):1252
Sipkova A, Szakova J, Hanc A, Tlustos P (2016) Mobility of mercury in soil as affected by soil physicochemical properties. J Soil Sediment 16(9):2234–2241
He CH, Arizono K, Ji HZ, Yakushiji Y, Zhang DZ, Huang KW, Ishibashi Y (2018) Spatial distribution characteristics of mercury in the soils and native earthworms (Bimastos parvus) of the leachate-contaminated zone around a traditional landfill. Sci Total Environ 636:1565–1576
Lindberg SE, Southworth G, Prestbo EM, Wallschlager D, Bogle MA, Price J (2005) Gaseous methyl- and inorganic mercury in landfill gas from landfills in Florida, Minnesota, Delaware, and California. Atmos Environ 39(2):249–258
Jovanov D, Vujic B, Vujic G (2018) Optimization of the monitoring of landfill gas and leachate in closed methanogenic landfills. J Environ Manage 216:32–40
Jones DL, Williamson KL, Owen AG (2006) Phytoremediation of landfill leachate. Waste Manage 26(8):825–837
de Diego A, Tseng CM, Dimov N, Amouroux D, Donard OFX (2001) Adsorption of aqueous inorganic mercury and methylmercury on suspended kaolin: influence of sodium chloride, fulvic acid and particle content. Appl Organomet Chem 15(6):490–498
Huang Y, Tang JC, Gai LS, Gong YY, Guan HW, He RZ, Lyu HH (2017) Different approaches for preparing a novel thiol-functionalized graphene oxide/Fe-Mn and its application for aqueous methylmercury removal. Chem Eng J 319:229–239
Beutel MW, Dent SR, Newcombe RL, Moller G (2019) Mercury removal from municipal secondary effluent with hydrous ferric oxide reactive filtration. Water Environ Res 91(2):132–143
Meer SR, Benson CH (2007) Hydraulic conductivity of geosynthetic clay liners exhumed from landfill final covers. J Geotech Geoenviron 133(5):550–563
Kumar H, Ganesan SP, Bordoloi S, Sreedeep S, Lin P, Mei GX, Garg A, Sarmah AK (2019) Erodibility assessment of compacted biochar amended soil for geo-environmental applications. Sci Total Environ 672:698–707
Musso TB, Francisca FM, Parolo ME, Roehl KE (2013) Potential use of calcareous mudstones in low hydraulic conductivity earthen barriers for environmental applications. Environ Technol 34(17):2465–2476
Paquette K, Helz G (1995) Solubility of cinnabar (RED HGS) and implications for mercury speciation in sulfidic waters. Water Air Soil Poll 80(1–4):1053–1056
Barrow NJ, Cox VC (1992) The effects of pH and chloride concentration on mercury adsorption. I. by goethite. Eur J Soil Sci 43:295–304
Xu JY, Kleja DB, Biester H, Lagerkvist A, Kumpiene J (2014) Influence of particle size distribution, organic carbon, pH and chlorides on washing of mercury contaminated soil. Chemosphere 109:99–105
Yin Y, Allen HE, Li Y, Huang CP, Sanders PF (1996) Adsorption of mercury (II) by soil: effects of pH, chloride, and organic matter. J Environ Qual 25:837–844
Wu CL, Cao Y, He CC, Dong ZB, Pan WP (2010) Study of elemental mercury re-emission through a lab-scale simulated scrubber. Fuel 89(8):2072–2080
Grilli S, Giordano A, Spagni A (2012) Stabilisation of biodried municipal solid waste fine fraction in landfill bioreactor. Waste Manage 32:1678–1684
Holley EA, McQuillan AJ, Craw D, Kim JP, Sander SG (2007) Mercury mobilization by oxidative dissolution of cinnabar (alpha-HgS) and metacinnabar (beta-HgS). Chem Geolog 240(3–4):313–325
Mikac N, Foucher D, Niessen S, Lojen S, Fischer JC (2003) Influence of chloride and sediment matrix on the extractability of HgS (cinnabar and metacinnabar) by nitric acid. Anal Bioanal Chem 377(7–8):1196–1201
Ravichandran M, Aiken GR, Reddy MM, Ryan JN (1998) Enhanced dissolution of cinnabar (mercuric sulfide) by dissolved organic matter isolated from the Florida Everglades. Environ Sci Technol 32(21):3305–3311
Zhang ZH, Zhao ZY, Fang QX, Qiao RH, Zhang T (2023) Extracellular polymeric substances enhance dissolution and microbial methylation of mercury sulfide minerals. Environ Sci Process Impacts 25(1):44–55
Paquette K, Helz G (1995) Solubility of cinnabar (red HgS) and implications for mercury speciation in sulfidic waters. Water Air Soil Pollut 80(1–4):1053–1056
Jew AD, Behrens SF, Rytuba JJ, Kappler A, Spormann AM, Brown GE (2013) Microbially enhanced dissolution of HgS in an acid mine drainage system in the California Coast Range. Geobiology 12(1):20–33
Jeong SJ, Nam AW, Yi SM, Kim JY (2015) Field assessment of semi-aerobic condition and the methane correction factor for the semi-aerobic landfills provided by IPCC guidelines. Waste Manage 36:197–203
Acknowledgements
This study was supported financially by the Environment Research and technology development Grant (3-1701 and JPMEERF20S20602), funded by the Ministry of the Environment, Japan. The authors appreciate the support greatly.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takahashi, F., Sano, A., Yanase, R. et al. 100-year simulation of mercury emissions from landfilled stabilized mercury waste. J Mater Cycles Waste Manag 25, 2654–2667 (2023). https://doi.org/10.1007/s10163-023-01691-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-023-01691-y