Abstract
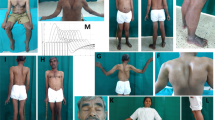
Background: Congenital myasthenic syndrome (CMS) is a heterogeneous group of rare disorders with impaired neuromuscular transmission caused by genetic defects, which is characterized by fatigable muscle weakness. Case presentation: Herein, we report a case of limb-girdle CMS (LG-CMS) in a 15-year-old Chinese girl with limb weakness and mild ptosis. The patient presented with well-defined clinical manifestations, muscle imaging, and electrophysiological features associated with CMS. On muscle biopsy, in addition to tubular aggregates identified, an extremely unusual pathological change of rimmed vacuoles in muscle fibers was observed. Whole-exome sequencing disclosed two novel heterozygous variants (c.14 T>A and c.581 T>C) in the human glutamine-fructose-6-phosphate transaminase 1 (GFPT1) gene, leading to the substitutions of phenylalanine to tyrosine (p.F5Y) and serine (p.F194S), respectively. Both variants were predicted to be likely pathogenic by SIFT, Polyphen-2, and Mutation Taster. Treatments with pyridostigmine bromide and albuterol produced a dramatic improvement. Conclusions: Collectively, molecular genetic analysis and muscle biopsy play crucial roles in the diagnosis of GFPT1-related LG-CMS with rimmed vacuoles (a rare phenotype of CMS) and have important implications for treatment decision.




Similar content being viewed by others
References
Engel AG (2012) Current status of the congenital myasthenic syndromes. Neuromuscul Disord 22(2):99–111. https://doi.org/10.1007/s11910-011-0234-7
Souza PV, Batistella GN, Lino VC, Pinto WB, Annes M, Oliveira AS (2016) Clinical and genetic basis of congenital myasthenic syndromes. Arq Neuropsiquiatr 74(9):750–760. https://doi.org/10.1590/0004-282X20160106
Eymard B, Hantai D, Estournet B (2013) Congenital myasthenic syndromes. Handb Clin Neurol 113:1469–1480. https://doi.org/10.1016/B978-0-444-59565-2.00016-2
Engel AG, Shen XM, Selcen D, Sine SM (2015) Congenital myasthenic syndromes: athogenesis, diagnosis, and treatment. Lancet Neurol 14(4):420–434. https://doi.org/10.1016/S1474-4422(14)70201-7
Parr J, Andrew MJ, Finnis M, Beeson D, Vincent A, Jayawant S (2014) How common is childhood myasthenia? The UK incidence and prevalence of autoimmune and congenital myasthenia. Arch Dis Child 99(6):539–542. https://doi.org/10.1136/archdischild-2013-304788
Rodriguez Cruz PM, Palace J, Beeson D (2014) Congenital myasthenic syndromes and the neuromuscular junction. Curr Opin Neurol 27(5):566–575. https://doi.org/10.1097/WCO.0000000000000134
Finlayson S, Spillane J, Kullmann DM, Howard R, Webster R, Palace J, Beeson D (2013) Slow channel congenital myasthenic syndrome responsive to a combination of fluoxetine and salbutamol. Muscle Nerve 47(2):279–282. https://doi.org/10.1002/mus.23534
Whittaker RG, Herrmann DN, Bansagi B, Hasan BA, Lofra RM, Logigian EL, Sowden JE, Almodovar JL, Littleton JT, Zuchner S, Horvath R, Lochmüller H (2015) Electrophysiologic features of SYT2 mutations causing a treatable neuromuscular syndrome. Neurology 85(22):1964–1971. https://doi.org/10.1212/WNL.0000000000002185
Selcen D, Shen XM, Milone M, Brengman J, Ohno K, Deymeer F, Finkel R, Rowin J, Engel AG (2013) GFPT1-myasthenia clinical, structural, and electrophysiologic heterogeneity. Neurology 81(4):370–378. https://doi.org/10.1212/WNL.0b013e31829c5e9c
Bauché S, Vellieux G, Sternberg D, Fontenille MJ, De Bruyckere E, Davoine CS, Brochier G, Messéant J, Wolf L, Fardeau M, Lacène E, Romero N, Koenig J, Fournier E, Hantaï D, Streichenberger N, Manel V, Lacour A, Nadaj-Pakleza A, Sukno S, Bouhour F, Laforêt P, Fontaine B, Strochlic L, Eymard B, Chevessier F, Stojkovic T, Nicole S (2017) Mutations in GFPT1-related congenital myasthenic syndromes are associated with synaptic morphological defects and underlie a tubular aggregate myopathy with synaptopathy. J Neurol 264(8):1791–1803. https://doi.org/10.1007/s00415-017-8569-x
Evangelista T, Hanna M, Lochmüller H (2015) Congenital myasthenic syndromes with predominant limb girdle weakness. J Neuromuscul Dis 2(Suppl 2):S21–S29. https://doi.org/10.3233/JND-150098
Huh SY, Kim HS, Jang HJ, Park YE, Kim DS (2012) Limb-girdle myasthenia with tubular aggregates associated with novel GFPT1 mutations. Muscle Nerve 46(4):600–604. https://doi.org/10.1002/mus.23451
Engel AG (2018) Congenital myasthenic syndromes in 2018. Curr Neurol Neurosci Rep 18(8):46. https://doi.org/10.1007/s11910-018-0852-4
Senderek J, Müller JS, Dusl M, Strom TM, Guergueltcheva V, Diepolder I, Laval SH, Maxwell S, Cossins J, Krause S, Muelas N, Vilchez JJ, Colomer J, Mallebrera CJ, Nascimento A, Nafissi S, Kariminejad A, Nilipour Y, Bozorgmehr B, Najmabadi H, Rodolico C, Sieb JP, Steinlein OK, Schlotter B, Schoser B, Kirschner J, Herrmann R, Voit T, Oldfors A, Lindbergh C, Urtizberea A, von der Hagen M, Hübner A, Palace J, Bushby K, Straub V, Beeson D, Abicht A, Lochmüller H (2011) Hexosamine biosynthetic pathway mutations cause neuromuscular transmission defect. Am J Hum Genet 88(2):162–172. https://doi.org/10.1016/j.ajhg.2011.01.008
Guergueltcheva V, Muller JS, Dusl M, Senderek J, Oldfors A, Lindbergh C, Maxwell S, Colomer J, Mallebrera CJ, Nascimento A, Vilchez JJ, Muelas N, Kirschner J, Nafissi S, Kariminejad A, Nilipour Y, Bozorgmehr B, Najmabadi H, Rodolico C, Sieb JP, Schlotter B, Schoser B, Herrmann R, Voit T, Steinlein OK, Najafi A, Urtizberea A, Soler DM, Muntoni F, Hanna MG, Chaouch A, Straub V, Bushby K, Palace J, Beeson D, Abicht A, Lochmüller H (2012) Congenital myasthenic syndrome with tubular aggregates caused by GFPT1 mutations. J Neurol 259(5):838–850. https://doi.org/10.1007/s00415-011-6262-z
Mihaylova V, Scola RH, Gervini B, Lorenzoni PJ, Kay CK, Werneck LC, Stucka R, Guergueltcheva V, von der Hagen M, Huebner A, Abicht A, Müller JS, Lochmüller H (2010) Molecular characterisation of congenital myasthenic syndromes in southern Brazil. J Neurol Neurosurg Psychiatry 81(9):973–977. https://doi.org/10.1136/jnnp.2009.177816
Eymard B, Ferreiro A, Ben Yaou R, Stojkovic T (2013) Muscle diseases with prominent joint contractures: Main entities and diagnostic strategy. Rev Neurol (Paris) 169(8–9):546–563. https://doi.org/10.1016/j.neurol.2013.07.005
Garg N, Yiannikas C, Hardy TA, Belaya K, Cheung J, Beeson D, Reddel SW (2016) Late presentations of congenital myasthenic syndromes: how many do we miss? Muscle Nerve 54(4):721–727. https://doi.org/10.1002/mus.25085
Chen Q, Muller JS, Pang PC, Laval SH, Haslam SM, Lochmüller H, Dell A (2015) Global N-linked glycosylation is not significantly impaired in myoblasts in congenital myasthenic syndromes caused by defective glutamine-fructose-6-phosphate transaminase 1 (GFPT1). Biomolecules 5(4):2758–2781. https://doi.org/10.3390/biom5042758
Nishikawa A, Mitsuhashi S, Miyata N, Nishino I (2017) Targeted massively parallel sequencing and histological assessment of skeletal muscles for the molecular diagnosis of inherited muscle disorders. J Med Genet 54(2):104–110. https://doi.org/10.1136/jmedgenet-2016-104073
Malicdan MC, Noguchi S, Nishino I (2007) Autophagy in a mouse model of distal myopathy with rimmed vacuoles or hereditary inclusion body myopathy. Autophagy 3(4):396–398. https://doi.org/10.4161/auto.4270
Lorenzon PJ, Scola RH, Kay CS, Werneck LC (2012) Congenital myasthenic syndrome: a brief review. Pediatr Neurol 46(3):141–148. https://doi.org/10.1016/j.pediatrneurol.2011.12.001
Muller JS, Stucka R, Neudecker S, Zierz S, Schmidt C, Huebner A, Lochmüller H, Abicht A (2005) An intronic base alteration of the CHRNE gene leading to a congenital myasthenic syndrome. Neurology 65(3):463–465. https://doi.org/10.1212/01.wnl.0000172346.26219.fd
Finsterer J (2019) Congenital myasthenic syndromes. Orphanet J Rare Dis 14(1):57. https://doi.org/10.1186/s13023-019-1025-5
Tsao CY (2016) Effective treatment with albuterol in DOK7 congenital myasthenic syndrome in children. Pediatr Neurol 54:85–87. https://doi.org/10.1016/j.pediatrneurol.2015.09.019
Acknowledgments
We thank Prof. Yanchen Xie and Dr. Hui Liu for their excellent work in revising and polishing this paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that no financial conflicts of interest exist in relation to the publication of this work.
Ethical standards
This work was performed in accordance with Ethical standard of Xi’an Gaoxin Hospital, Xi’an Medical College, and approved by the Ethics Committees of Xi'an Gaoxin Hospital.
Informed consent
Informed consent was obtained from the patient for publication of this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, Y., Xiong, T., Lei, G. et al. Novel compound heterozygous variants in the GFPT1 gene leading to rare limb-girdle congenital myasthenic syndrome with rimmed vacuoles. Neurol Sci 42, 3485–3490 (2021). https://doi.org/10.1007/s10072-020-05021-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-05021-0