Abstract
Objectives
Extrahepatic tryptophan (Trp)-kynurenine (Kyn) metabolism via indoleamine 2,3-dioxygenase 1 (IDO1) induction was found to be associated with intrinsic immune regulation. However, the Trp-Kyn metabolism–associated immune regulation in dermatomyositis (DM) remains unknown. Therefore, we aimed to investigate the clinical relevance of the Trp-Kyn metabolism via IDO1 induction in DM.
Methods
Liquid chromatography-mass spectrometry (HPLC–MS) was used to examine the serum Kyn and Trp concentrations in DM. In addition, we used X-tile software to determine the optimal cutoff value of the Kyn/Trp ratio, a surrogate marker for Trp-Kyn metabolism. Spearman analysis was performed to evaluate the association of Trp-Kyn metabolism with muscle enzymes and inflammatory markers.
Results
DM patients had significantly higher serum Kyn/Trp ratio (× 10−3) when compared with the healthy controls. The serum Kyn/Trp ratio was positively correlated with the levels of muscle enzymes and inflammatory markers. In addition, the serum Kyn/Trp ratio significantly decreased (36.89 (26.00–54.00) vs. 25.00 (18.00–37.00), P = 0.0006) after treatment. DM patients with high serum Kyn/Trp ratio had a significantly higher percentage of muscle weakness symptoms (62.5% vs. 20.0%, P = 0.019) and higher levels of LDH (316.0 (236.0–467.0) vs. 198.0 (144.0–256.0), P = 0.004) and AST (56.5 (35.0–92.2) vs. 23.0 (20.0–36.0), P = 0.002)) than those with low serum Kyn/Trp ratio. Multiple Cox regression analyses identified ln(Kyn/Trp) (HR 4.874, 95% CI 1.105–21.499, P = 0.036) as an independent prognostic predictor of mortality in DM.
Conclusions
DM patients with enhanced Trp-Kyn metabolism at disease onset are characterized by more severe disease status and poor prognosis. Intrinsic immune regulation function via enhanced Trp-Kyn metabolism by IDO1 induction may be a potential therapeutic target in DM.
Key Points • HPLC–MS identified increased serum Kyn/Trp ratio in DM patients, which positively correlated with levels of muscle enzymes and inflammatory markers and was downregulated upon treatment. • Cox regression analyses identified ln(Kyn/Trp) as an independent prognostic predictor of mortality in DM. • Monitoring intrinsic immune regulation function should be considered a potential therapeutic target in DM patients. |
Similar content being viewed by others
Introduction
Dermatomyositis (DM) is an autoimmune inflammatory disorder characterized by the presence of pathognomonic skin lesions (heliotrope rash, Gottron’s papules/sign, poikiloderma, etc.) with or without muscular and extra-muscular manifestations (primarily lung involvement, followed by heart, esophagus, gastrointestinal tract, etc.) [1]. Disease activity is reflected by levels of muscle enzymes or inflammatory markers. Moreover, interstitial lung disease (ILD) and malignancy are frequently correlated with disease severity and prognosis [2,3,4].
Autoimmune-mediated pathological inflammation plays a crucial role in the pathogenesis of DM [5]. Corresponding anti-inflammatory therapeutic strategies, including glucocorticoids and immunosuppressants, were widely used in clinical practice [6, 7]. However, the immune system itself can limit excessive inflammatory responses in normal physiology, termed “immune regulation.” Immune homeostasis is a highly dialectical situation comprising immune regulation and inflammation. They interlace with each other and once the balance is broken, disease occurs. The function of immune regulation would have also altered under the disease conditions, and we propose that the intrinsic immune regulation function should provoke new ideas for disease monitoring and treatment.
The extrahepatic tryptophan (Trp)-kynurenine (Kyn) metabolism is a crucial metabolic foundation underlying the intrinsic immune regulation function [8]. In the later phase of inflammation, IFN-γ induces indoleamine 2,3-dioxygenase 1 (IDO1), the rate-limiting enzyme for Trp-Kyn metabolism, in extensive cells (monocytes, macrophages, plasmacytoid dendritic cells, etc.) converting Trp to Kyn to drive the intrinsic immune regulation function. Impairment in IDO1 induction exacerbates the underlying inflammatory pathology, and reversing this defect would cause improvement [9]. The serum Kyn/Trp ratio equals to IDO1 activity and allows an estimation for level of Trp-Kyn metabolism, essentially serving as an indicator of intrinsic immune functional status. Trp-Kyn metabolism increased in many diseases with chronic immune activation, such as chronic inflammatory, autoimmune, and allergic diseases, as well as cancer and atherosclerosis [10]. Enhanced extrahepatic Trp-Kyn metabolism was reported to be associated with poor prognosis in cancer or disease activity in chronic inflammatory disorders [11, 12]. Collectively, we hypothesized that the activity of Trp-Kyn metabolism reflects the disease severity and may affect the prognosis in DM.
Interestingly, it has been reported that Trp metabolism is significantly altered in DM [13]. However, further clinical information about its downstream Trp-Kyn metabolism is limited. Therefore, we measured the serum levels of Trp and Kyn and calculated the serum Kyn/Trp ratio to determine the association of Trp-Kyn metabolism with clinical characteristics, treatment, and clinical outcomes in DM patients.
Patients and methods
Patients
This was a single-center, retrospective, observational study. From January 2017 to September 2020, we enrolled 57 newly diagnosed and treatment-naïve DM patients admitted to the Department of Dermatology at Shanghai Rui Jin Hospital. We also included 10 age- and sex-matched healthy controls (HCs). Classic DM (CDM) or clinically amyopathic DM (CADM) was diagnosed based on Bohan and Peter’s criteria or modified Sontheimer’s definitions [14, 15]. Treatment-naïve DM was defined as having no history of corticosteroid or immunosuppressant use before admission. All patients completed an initial screening before enrollment to evaluate organ involvement and tumor. ILD was diagnosed based on characteristic abnormalities on high-resolution computed tomography (HRCT) and clinical presentations, with the exclusion of pulmonary infections determined by two experienced pulmonologists. All DM patients were divided into two groups according to the optimal cutoff value of the serum Kyn/Trp ratio (× 10−3) determined by X-tile software [16], the high serum Kyn/Trp ratio group (> 38.50, n = 32) and the low serum Kyn/Trp ratio group (≤ 38.50, n = 25). The Ruijin Hospital Ethics Committee approved this study according to Confidential Agreement No. 2016 (105), and informed consent was obtained from all participants.
Data collection
Patient general characteristics and clinical information were retrieved from hospital electronic medical records by a dedicated person from the Department of Information Technology. All patients were followed up until the endpoint of this study (June 30, 2021), and data were collected without any missing information. Survival time was defined as the time to death or censoring date from the hospital admission date. Electronic medical records were used to collect and collate the corresponding clinical characteristics and laboratory data. Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) was performed by two dermatologists (H.C. and D.W.), as reported previously [17]. The serum Kyn/Trp ratio was calculated to represent a surrogate marker for Trp-Kyn metabolism level, and X-tile software was used to determine the optimal cutoff value.
Measurement of serum tryptophan and kynurenine concentrations
Sera were separated by centrifugation at 3000 rpm for 5 min at room temperature and stored frozen at − 80 °C away from light until use. The serum concentrations of Kyn and Trp were quantified using an Agilent 1100 high-performance liquid chromatography-mass spectrometer (HPLC–MS) system. Serum samples were pre-treated using protein precipitation. A 10 µL aliquot of the supernatant was injected into the HPLC–MS system equipped with an Agilent ZORBAX SB-C18 column (2.1 mm × 150 mm, 5 µm) to acquire the mass spectra peak area under the selected ion monitor (SIM) mode. The serum concentrations of Trp and Kyn were evaluated using an internal standard calibration curve. The calibration curve was established as follows. Taking the concentrations of Trp or Kyn calibration samples as the X-axis, the ratio of the mass spectra peak area between Trp or Kyn to the internal standard (metronidazole) as the Y-axis, the regression equation of concentration and mass spectra peak area was obtained using the least squares method.
Statistical analysis
Continuous variables with normal distribution are reported as the mean ± standard deviation (SD). Continuous variables with a skewed distribution are reported as medians with interquartile ranges (IQRs). Categorical variables are presented as frequencies (percentages). We used the Student’s t-test for continuous variable comparisons or the Mann–Whitney U test for non-parametric continuous variable comparisons. Categorical variables were compared using the chi-squared test or Fisher’s exact test. The results were corrected for multiple comparisons using Benjamin-Hochberg false discovery rate (FDR). Spearman’s rank correlation analysis was used for non-parametric correlation analysis. Survival analysis was performed using the Kaplan–Meier method and log-rank test. Cox proportional-hazards regression analysis was used for the assessment of prediction of mortality. The Wilcoxon signed rank-sum test was used to compare the data before and after medication. Statistical significance was set at P < 0.05. Statistical analysis and graphing were performed using GraphPad Prism 8 software and R software (R version 4.0.4, http://www.R-project.org).
Results
Patient characteristics
In our cohort study, the general information of DM patients is summarized in Supplementary Table 1, including 24 (42.1%) cases of CDM and 33 (57.9%) cases of CADM. The average age of onset was 52.39 ± 15.6 years old (age range 20–79 years). Eighteen (31.6%) were male (9 CDM and 9 CADM), and 39 (68.4%) were female (15 CDM and 24 CADM). Twenty-nine (50.9%) patients had ILD, and 16 (28.1%) had malignancies, including breast, lung, and nasopharyngeal cancers. Among them, CDM patients had a higher incidence of malignancy than those with CADM (21% vs. 7%, respectively; P = 0.065).
Elevated serum Kyn/Trp ratio in dermatomyositis
The serum Kyn/Trp ratio (× 10−3) was significantly higher in all treatment–naïve DM patients than in healthy controls (HCs) (41.73 (31.78–62.15) vs. 27.50 (21.75–32.75), respectively; P = 0.0101) (Fig. 1A). In contrast, the serum Trp level (µM) was numerically lower in DM patients compared with HCs (32.55 (24.12–47.21) vs. 35.77 (31.75–51.4), respectively; P = 0.2389) (Fig. 1B). The serum Kyn level (µM) was numerically higher in DM patients compared with HCs (1.344 (0.902–1.964) vs. 0.9795 (0.736–1.813), respectively; P = 0.1922) (Fig. 1C). Though the differences in tryptophan and kynurenine levels did not reach statistical significance.
Comparison of serum Kyn/Trp ratio (A), tryptophan (B), and kynurenine (C) levels between DM patients and healthy controls. DM* comprises clinically amyopathic dermatomyositis (CADM) and classic dermatomyositis (CDM). Comparisons between two groups were assessed by the Mann–Whitney U test. Bars represent medians ± interquartile range. Abbreviations: Kyn/Trp, kynurenine to tryptophan; HC, healthy control; DM, dermatomyositis
Associations of serum Kyn/Trp ratio with disease activity indices
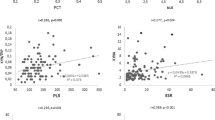
In terms of serological indices, the serum Kyn/Trp ratio was found to be positively correlated with muscle enzymes, such as creatine kinase (CK) (rs = 0.4167), lactate dehydrogenase (LDH) (rs = 0.4961), aspartate aminotransferase (AST) (rs = 0.5313), myoglobin (rs = 0.4945), and CK isoenzyme-MB (CK-MB) mass (rs = 0.4155), and clinical inflammatory indicators, such as C-reactive protein (CRP) (rs = 0.4679), serum ferritin (SF) (rs = 0.3390), β2-microglobulin (β2-MG) (rs = 0.6960), neutrophil/lymphocyte ratio (NLR) (rs = 0.3833), and erythrocyte sedimentation rate (ESR) (rs = 0.3731). However, the serum Kyn/Trp ratio was negatively correlated with complement C3 (rs = − 0.2712) (Fig. 2A-K). There was no association between the serum Kyn/Trp ratio and CDASI (activity or damage) scoring system.
Serum Kyn/Trp ratio correlates with the serological indices in DM patients. A–E Correlation of serum Kyn/Trp ratio with CK, LDH, AST, myoglobin, and CM-MB mass (n = 57). F–K Correlation of serum Kyn/Trp ratio with CRP (n = 55), ferritin (n = 56), β2-MG (n = 38), NLR (n = 57), ESR (n = 54), and C3 (n = 54). Spearman’s rank correlation analysis was used for non-parametric correlation analysis. Abbreviations: Kyn/Trp, kynurenine to tryptophan; CK, creatine kinase; LDH, lactate dehydrogenase; AST, aspartate aminotransferase; CK-MB, creatine kinase isoenzyme-MB; CRP, C-reactive protein; β2-MG: beta-2-microglobulin; NLR, neutrophil/ lymphocyte ratio; ESR, erythrocyte sedimentation rate; C3, complement C3
Patient characteristics according to serum Kyn/Trp ratio
An optimal cutoff tool named “X-tile software” was used to stratify DM patients into high (serum Kyn/Trp ratio (× 10−3) > 38.50, n = 32) and low (serum Kyn/Trp ratio (× 10−3) ≤ 38.50, n = 25) tryptophan metabolism group. Patient key characteristics are listed in Table 1 (complete characteristics are reported in Supplementary Table 3). No statistical differences in age at diagnosis, sex, and body mass index (BMI) were found between the two groups.
DM patients with high serum Kyn/Trp ratio had a significantly higher frequency of CDM (62.5% vs. 16%, P = 0.008) and a lower frequency of CADM (37.5% vs. 84.0%, P = 0.008) than those with low serum Kyn/Trp ratio, in concert with a higher percentage of muscle weakness symptoms (62.5% vs. 20.0%, P = 0.019) and higher levels of LDH (316.0 (236.0–467.0) vs. 198.0 (144.0–256.0), P = 0.004), AST (56.5 (35.0–92.2) vs. 23.0 (20.0–36.0), P = 0.002), CK-MB (3.9 (1.4–14.4) vs. 1.2 (0.9–2.2), P = 0.024), and myoglobin (90.6 (28.9–334.0) vs. 27.4 (18.1–38.3), P = 0.008).
Additionally, DM patients with high serum Kyn/Trp ratio had higher levels of inflammatory markers than those with low serum Kyn/Trp ratio, including NLR (3.2 (2.5–5.5) vs. 2.1 (1.4–3.2), P = 0.019), CRP (1.0 (0.3–3.6) vs. 0.3 (0.2–0.4), P = 0.041), and β2-MG (3022 ± 823 vs. 2247 ± 667, P = 0.019).
Survival analysis according to serum Kyn/Trp ratio
In our cohort, the cumulative survival rate was 86.19%. Survival analysis showed that DM patients with high Trp-Kyn metabolism had worse overall survival than those with low Trp-Kyn metabolism (log-rank P = 0.0033) (Fig. 3). Patients with high Trp-Kyn metabolism had a higher frequency of comorbidities, including interstitial lung disease or malignancy, and the main causes of death were respiratory failure due to interstitial lung disease (3/9, 33.3%) or the exacerbation of malignancy (6/9, 66.7%). Cox proportional-hazards regression model showed that ln(Kyn/Trp) was an independent predictor of mortality in the cohort under investigation. After adjusting for BMI, WBC, malignancy, hypertension, and diabetes mellitus, ln(Kyn/Trp) (HR 4.874, 95% CI 1.105–21.499, P = 0.036) was still an independent predictor of mortality (Table 2).
Impact of dermatomyositis treatment on serum Kyn/Trp ratio
The effect of DM medication on the serum Kyn/Trp ratio was assessed in 23 individuals that achieved disease remission with serum samples available during follow-up. The mean duration of treatment was 3.30 ± 1.52 months, ranging from 1 to 7 months. Therapeutic strategies for DM are summarized in Supplementary Table 2. After comprehensive treatment, the serum Kyn/Trp ratio decreased significantly (36.89 (26.00–54.00) vs. 25.00 (18.00–37.00), P = 0.0006) (Fig. 4A), and the level of serum Trp (µM) concentration increased significantly (29.19 (22.44–44.55) vs. 61.76 (48.05–68.98), P < 0.0001) (Fig. 4B). However, the changes in serum Kyn levels were not statistically significant (P = 0.2467, Fig. 4C).
Serum tryptophan and kynurenine levels as well as Kyn/Trp ratio before and after treatment in DM patients (n = 23). The Wilcoxon signed rank-sum test was used to compare the data before and after medication. Abbreviations: Pre-Tre, pre-treatment; Post-Tre, post-treatment; Kyn/Trp, kynurenine to tryptophan
Discussion
The essential role of immunity is “self–nonself” discrimination [18]. Inflammation can be concluded as the activation of immune cells during the defense and clearance of “nonself.” However, they were always limited and restrained by intrinsic immune regulation function in a feedback loop to restore immune homeostasis. Trp-Kyn metabolism level is an important indicator for such intrinsic immune functional status. In this study, we explored the clinical association of the Trp-Kyn metabolism via IDO1 induction in DM and demonstrated that (1) an increased serum Kyn/Trp ratio in DM correlated with disease activity; (2) Cox regression analyses identified ln(Kyn/Trp) as an independent prognostic predictor of mortality; and (3) serum Kyn/Trp ratio decreased upon treatment.
In previous basic research, it has been identified that higher Trp oxidation in the muscle of patients with polymyositis contributes to the mitochondrial proteins’ alteration and mitochondrial dysfunction, which is a common pathway shared in muscle disease [19]. Later, a bio-informatic metabolomic study identified the aminoacyl-tRNA biosynthesis, Trp biosynthesis, and metabolism were perturbed pathways in DM and showed that the decreased Trp levels had diagnostic values for DM [13]. In this study, the clinical aspect of Trp metabolism in DM was considered through the disease activity, treatment, and prognosis.
Trp-Kyn metabolism via IDO1 induction has also been reported with clinical relevance in other autoimmune diseases. For example, in SLE patients, enhanced Trp-Kyn metabolism correlated with disease activity and severe fatigue and could predict disease activation [20,21,22,23]. Elevated IDO1 activity and increased regulatory T cells have been discovered and correlated with disease severity and interferon-positive signatures in patients with Sjogren’s syndrome [24, 25]. Moreover, serum IDO1 activity was confirmed to be increased in psoriasis, where immune cells from patients with psoriasis are defective in inducing IDO1 upregulation in response to inflammatory stimuli [26].
In this study, we observed that patients with high Kyn/Trp ratio had more muscle involvement (CADM vs. CDM, muscle enzymes), which is in congruence with previous basic research, indicating that an enhanced extrahepatic Trp-Kyn metabolic pathway and implying that the subsequent intrinsic immune regulation function could play a role in muscle tissue damage and repairment. It is reasonable to speculate that Trp-Kyn metabolism may also contribute to the robustness of the tissue reconstruction system by maintaining immune homeostasis [27]. Skeletal muscle is the largest reservoir of amino acids in the body; it has been reported that aminoacyl-tRNA, phenylalanine, and tyrosine biosynthesis pathways were also altered in DM. Muscle metabolism–related changes in immune function may be a new therapeutic target for DM.
What’s more, the serum Kyn/Trp ratio correlated with disease activity indicators. However, there was no correlation between the serum Kyn/Trp ratio and CDASI (activity or damage). We hypothesized that the CDASI indicates the severity of skin lesions while the Kyn/Trp ratio represents the whole level of immune status. Given the alterations of Trp-Kyn metabolism before and after treatment, the level of ln(Kyn/Trp) showed potential prognostic value for survival. This would implicate the underlying impairment in the intrinsic immune regulation function in patients with DM.
Although this is an observational study, this research has important implications. Once inflammation was initiated, the intrinsic immune regulation function also occurred (Fig. 5A). It may be driven by extrahepatic Trp-Kyn metabolism via IDO1 induction to limit the excessive inflammatory reactions (Fig. 5B) [28]. Such findings can be explained as “Trp depletion” and “Kyn production” [29]. The former leads to the inhibition of effector immune cells via the general control nonderepressible two kinase (GCN2K) signaling pathway. The latter activates the aromatic hydrocarbon receptor (AhR) signaling pathway, which induces anti-inflammatory signaling [30]. A Trp-Kyn metabolism via the IDO1 induction comic is shown in Supplementary Fig. 1. So we proposed that other than “anti-inflammation” therapeutic strategies, intrinsic immune regulation function would be a promising pipeline for the treatment and surveillance of DM.
Immune homeostasis and local immune regulation via the tryptophan-kynurenine metabolic pathway via IDO1 induction. A Inflammation and immune regulation co-occur to maintain dynamic immune homeostasis. B IDO1 induction upon inflammatory stimuli drives the extrahepatic tryptophan-kynurenine metabolic pathway to play a role in local immune regulation. ① Pro-inflammatory stimuli; ② IDO1 induction; ③ enhanced extrahepatic tryptophan-kynurenine pathway; ④ altered phenotype of immune cells; ⑤ immune tolerance and immune suppression; ⑥ immune homeostasis. Abbreviations: Trp, tryptophan; Kyn, kynurenine; IFN-γ, interferon-gamma; IDO1, indoleamine 2,3-dioxygenase 1; M1, M1 macrophages; Th1, CD4 + T helper 1; Treg, regulatory T cell
The current first-line treatment of DM is still mainly empirical glucocorticoids with immunosuppressants [31]; due to its side-effects, new approaches are still in need. Trp-Kyn metabolism could be an essential target for restoration of intrinsic immune regulation function with potentially translational implications and may be a source of future work. First, regular aerobic exercise of a certain intensity can promote muscle recovery by altering the Kyn pathway [32]. Intensive aerobic exercise and resistance training can reduce disease activity and promote muscular improvement in DM [33, 34]. Shifting the Kyn pathway after exercise could be one reason that non-pharmacological treatment exercises effectively treat DM. Second, changes in food composition and lifestyle, including physical activity and weight loss, have been shown to influence Trp availability [35, 36]. The regulation of Trp content showed clinical potential in the prevention and treatment of immune-related disorders, which may also have therapeutic potential in DM [37]. Recently, IDO1 inhibitors have been developed and entered the clinical assessment phase and show promise for cancer therapy by strengthening the anti-tumor immune response [38]. Regulating IDO1 expression and activity may be a potential therapeutic strategy for DM.
Overall, we added additional evidence to the literature pool on recognizing another potential marker for clinical outcome prediction among patients with DM. A better understanding of such a signaling pathway could help physicians to differentiate between the DM subtypes. More importantly, our study showed that intrinsic immune regulation function is also of paramount importance for immune homeostasis and could also be a target for therapy in DM.
Importantly, our investigation has some limitations that require discussion. First, the presented data demonstrate associations rather than causal relationships or mechanistic insights. Second, without directly assessing IDO1 expression in serum, we can only hypothesize that increased Trp-Kyn metabolism in DM may associate with altered intrinsic immune regulation function. Interventional clinical studies are needed to determine whether modification of intestinal tryptophan pathways affects the severity of DM. Third, the sample size of our investigation is rather limited. Further studies with larger sample sizes and prospective cohorts are required to confirm our findings. Fourth, other disease-specific outcome measures such as physician global disease activity scores, manual muscle testing, or patient reported outcomes had not been done to comprehensively evaluate the severity of the disease.
In the present study, we determined an immunometabolic feature of enhanced Trp-Kyn metabolism and its clinical relevance in treatment-naïve adult DM patients. The role of immune regulation via the Trp-Kyn pathway triggered by IDO1 induction in DM needs to be further investigated for its clinical potential in the future. Monitoring and intervention in the functional state of immune regulation in the human body need to be considered as a potential therapeutic target in DM.
Data availability
All data of this study are available in the article or uploaded as online supplementary material.
Change history
28 July 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10067-022-06317-6
References
Lundberg IE, Fujimoto M, Vencovsky J, Aggarwal R, Holmqvist M, Christopher-Stine L et al (2021) Idiopathic inflammatory myopathies. Nat Rev Dis Primers 7(1):86. https://doi.org/10.1038/s41572-021-00321-x
Lega JC, Reynaud Q, Belot A, Fabien N, Durieu I, Cottin V (2015) Idiopathic inflammatory myopathies and the lung. Eur Respir Rev 24(136):216–238. https://doi.org/10.1183/16000617.00002015
Wolstencroft PW, Fiorentino DF (2018) Dermatomyositis clinical and pathological phenotypes associated with myositis-specific autoantibodies. Curr Rheumatol Rep 20(5):28. https://doi.org/10.1007/s11926-018-0733-5
Barnes BE, Mawr B (1976) Dermatomyositis and malignancy. A review of the literature. Ann Intern Med 84(1):68–76. https://doi.org/10.7326/0003-4819-84-1-68
Thompson C, Piguet V, Choy E (2018) The pathogenesis of dermatomyositis. Br J Dermatol 179(6):1256–1262. https://doi.org/10.1111/bjd.15607
Gordon PA, Winer JB, Hoogendijk JE, Choy EH (2012) Immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database Syst Rev 2012(8):Cd003643. https://doi.org/10.1002/14651858.CD003643.pub4
Waldman R, DeWane ME, Lu J (2020) Dermatomyositis: diagnosis and treatment. J Am Acad Dermatol 82(2):283–296. https://doi.org/10.1016/j.jaad.2019.05.105
Cervenka I, Agudelo LZ, Ruas JL (2017) Kynurenines: tryptophan’s metabolites in exercise, inflammation, and mental health. Sci 357(6349). https://doi.org/10.1126/science.aaf9794
Mellor AL, Lemos H, Huang L (2017) Indoleamine 2,3-dioxygenase and tolerance: where are we now? Front Immunol 8:1360. https://doi.org/10.3389/fimmu.2017.01360
Huang L, Baban B, Johnson BA 3rd, Mellor AL (2010) Dendritic cells, indoleamine 2,3 dioxygenase and acquired immune privilege. Int Rev Immunol 29(2):133–155. https://doi.org/10.3109/08830180903349669
Hornyák L, Dobos N, Koncz G, Karányi Z, Páll D, Szabó Z et al (2018) The role of indoleamine-2,3-dioxygenase in cancer development, diagnostics, and therapy. Front Immunol 9:151. https://doi.org/10.3389/fimmu.2018.00151
Ketelhuth DFJ (2019) The immunometabolic role of indoleamine 2,3-dioxygenase in atherosclerotic cardiovascular disease: immune homeostatic mechanisms in the artery wall. Cardiovasc Res 115(9):1408–1415. https://doi.org/10.1093/cvr/cvz067
Zhang T, Xu J, Liu Y, Liu J (2019) Metabolomic profiling for identification of potential biomarkers in patients with dermatomyositis. Metabolomics 15(5):77. https://doi.org/10.1007/s11306-019-1539-9
Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (first of two parts). N Engl J Med 292(7):344–347. https://doi.org/10.1056/nejm197502132920706
Sontheimer RD (2002) Would a new name hasten the acceptance of amyopathic dermatomyositis (dermatomyositis siné myositis) as a distinctive subset within the idiopathic inflammatory dermatomyopathies spectrum of clinical illness? J Am Acad Dermatol 46(4):626–636. https://doi.org/10.1067/mjd.2002.120621
Camp RL, Dolled-Filhart M, Rimm DL (2004) X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10(21):7252–7259. https://doi.org/10.1158/1078-0432.Ccr-04-0713
Chen M, Quan C, Diao L, Xue F, Xue K, Wang B et al (2018) Measurement of cytokines and chemokines and association with clinical severity of dermatomyositis and clinically amyopathic dermatomyositis. Br J Dermatol 179(6):1334–1341. https://doi.org/10.1111/bjd.17079
Pradeu T (2019) Immunology and individuality. Elife 8. https://doi.org/10.7554/eLife.47384
Sunitha B, Gayathri N, Kumar M, Keshava Prasad TS, Nalini A, Padmanabhan B et al (2016) Muscle biopsies from human muscle diseases with myopathic pathology reveal common alterations in mitochondrial function. J Neurochem 138(1):174–191. https://doi.org/10.1111/jnc.13626
Furuzawa-Carballeda J, Lima G, Jakez-Ocampo J, Llorente L (2011) Indoleamine 2,3-dioxygenase-expressing peripheral cells in rheumatoid arthritis and systemic lupus erythematosus: a cross-sectional study. Eur J Clin Invest 41(10):1037–1046. https://doi.org/10.1111/j.1365-2362.2011.02491.x
Pertovaara M, Hasan T, Raitala A, Oja SS, Yli-Kerttula U, Korpela M et al (2007) Indoleamine 2,3-dioxygenase activity is increased in patients with systemic lupus erythematosus and predicts disease activation in the sunny season. Clin Exp Immunol 150(2):274–278. https://doi.org/10.1111/j.1365-2249.2007.03480.x
Widner B, Sepp N, Kowald E, Ortner U, Wirleitner B, Fritsch P et al (2000) Enhanced tryptophan degradation in systemic lupus erythematosus. Immunobiology 201(5):621–630. https://doi.org/10.1016/s0171-2985(00)80079-0
Åkesson K, Pettersson S, Ståhl S, Surowiec I, Hedenström M, Eketjäll S et al (2018) Kynurenine pathway is altered in patients with SLE and associated with severe fatigue. Lupus Sci Med 5(1):e000254. https://doi.org/10.1136/lupus-2017-000254
Maria NI, van Helden-Meeuwsen CG, Brkic Z, Paulissen SM, Steenwijk EC, Dalm VA et al (2016) Association of increased Treg cell levels with elevated indoleamine 2,3-dioxygenase activity and an imbalanced kynurenine pathway in interferon-positive primary Sjogren’s syndrome. Arthritis Rheumatol 68(7):1688–1699. https://doi.org/10.1002/art.39629
Pertovaara M, Raitala A, Uusitalo H, Pukander J, Helin H, Oja SS et al (2005) Mechanisms dependent on tryptophan catabolism regulate immune responses in primary Sjögren’s syndrome. Clin Exp Immunol 142(1):155–161. https://doi.org/10.1111/j.1365-2249.2005.02889.x
Llamas-Velasco M, Bonay P, Jose Concha-Garzon M, Corvo-Villen L, Vara A, Cibrian D et al (2017) Immune cells from patients with psoriasis are defective in inducing indoleamine 2,3-dioxygenase expression in response to inflammatory stimuli. Br J Dermatol 176(3):695–704. https://doi.org/10.1111/bjd.14779
Truchetet ME, Pradeu T (2018) Re-thinking our understanding of immunity: robustness in the tissue reconstruction system. Semin Immunol 36:45–55. https://doi.org/10.1016/j.smim.2018.02.013
Melhem NJ, Taleb S (2021) Tryptophan: from diet to cardiovascular diseases. Int J Mol Sci. 22(18). https://doi.org/10.3390/ijms22189904
Fiore A, Murray PJ (2021) Tryptophan and indole metabolism in immune regulation. Curr Opin Immunol 70:7–14. https://doi.org/10.1016/j.coi.2020.12.001
Krupa A, Kowalska I (2021) The kynurenine pathway-new linkage between innate and adaptive immunity in autoimmune endocrinopathies. Int J Mol Sci 22(18). https://doi.org/10.3390/ijms22189879
Pipitone N, Salvarani C (2020) Up-to-date treatment and management of myositis. Curr Opin Rheumatol 32(6):523–527. https://doi.org/10.1097/bor.0000000000000745
Martin KS, Azzolini M, Lira RJ (2020) The kynurenine connection: how exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. Am J Physiol Cell Physiol 318(5):C818–C830. https://doi.org/10.1152/ajpcell.00580.2019
Alexanderson H (2016) Physical exercise as a treatment for adult and juvenile myositis. J Intern Med 280(1):75–96. https://doi.org/10.1111/joim.12481
Voet NB, van der Kooi EL, van Engelen BG, Geurts AC (2019) Strength training and aerobic exercise training for muscle disease. Cochrane Database Syst Rev 12(12):CD003907. https://doi.org/10.1002/14651858.CD003907.pub5
Metcalfe AJ, Koliamitra C, Javelle F, Bloch W, Zimmer P (2018) Acute and chronic effects of exercise on the kynurenine pathway in humans - a brief review and future perspectives. Physiol Behav 194:583–587. https://doi.org/10.1016/j.physbeh.2018.07.015
Strasser B, Berger K, Fuchs D (2015) Effects of a caloric restriction weight loss diet on tryptophan metabolism and inflammatory biomarkers in overweight adults. Eur J Nutr 54(1):101–107. https://doi.org/10.1007/s00394-014-0690-3
Lin J, Sun-Waterhouse D, Cui C (2021) The therapeutic potential of diet on immune-related diseases: based on the regulation on tryptophan metabolism. Crit Rev Food Sci Nutr. 1–19. https://doi.org/10.1080/10408398.2021.1934813
Tang K, Wu YH, Song Y, Yu B (2021) Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J Hematol Oncol 14(1):68. https://doi.org/10.1186/s13045-021-01080-8
Acknowledgements
We thank Professor Jun Wu (NHC Key Lab of Reproduction Regulation, Shanghai Institute of Planned Parenthood Research, Medical School, Fudan University, Shanghai, China) and Dr. Jian Li (Department of Clinical Research Center, Rui Jin Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China) for providing technical assistance.
Funding
This study was supported by the National Natural Science Foundation of China (81573037, 81872523, 82073432), Clinical Research Plan of SHDC (16CR3084B), National Key Clinical Specialty (2012649), Shanghai Municipal Education Commission–Gaofeng Clinical Medicine Grant Support (20172009), Science and Technology Commission of Shanghai Municipality (134119a6100), and Shanghai Yiyuan Rising Star Outstanding Young Medical Talents (2019).
Author information
Authors and Affiliations
Contributions
Study conception and design: D.W., J.Z., Y.L., and H.C.; data acquisition: D.W. and Y.L.; funding acquisition: H.C.; data analysis and interpretation: D.W., C.M., S.C., S.Z., Y.C., and Q.Z.; supervision: J.Z., Y.L., and H.C.; visualization: K.X. and F.X.; writing—original draft preparation: D.W.; writing—review and editing: X.C., M.Z., H.L., J.Z., Y.L., and H.C.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ruijin Hospital Ethics Committee approved this study according to Confidential Agreement No. 2016 (105), and informed consent was obtained from all participants. This study was conducted according to the principles of the Declaration of Helsinki.
Disclosures
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Key Points has been updated.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, D., Chen, M., Chen, S. et al. Enhanced tryptophan-kynurenine metabolism via indoleamine 2,3-dioxygenase 1 induction in dermatomyositis. Clin Rheumatol 41, 3107–3117 (2022). https://doi.org/10.1007/s10067-022-06263-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-022-06263-3