Abstract
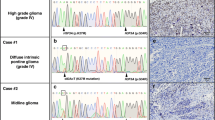
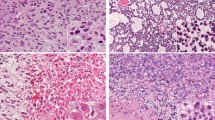
A recurrent glycine-to-arginine/valine alteration at codon 34 (G34R/V) within H3F3A, a gene that encodes the replication-independent histone variant H3.3, reportedly occurs exclusively in pediatric glioblastomas. However, the clinicopathological and biological significances of this mutation have not been completely elucidated; especially, no such data exist for tumor samples from Japanese patients. We analyzed 411 consecutive glioma cases representing patients of all ages. Our results demonstrated that 14 patients (3.4%) harbored H3F3A mutations, of which four had G34R mutations and 10 had K27M mutations. G34R-mutant tumors were located in the parietal region in two patients and the basal ganglia in one patient. One patient showed multi-lobular extension similar to the pattern observed in gliomatosis cerebri. Regarding neuroradiological features, intratumoral calcification was evident in two cases and all cases showed no or scarce contrast enhancement on MRI. Histopathologically, the four G34R-mutant cases included three glioblastomas and one astroblastoma. We have also investigated alterations in histone methylation including H3K27me3, H3K9me3, and H3K4me3 in G34R-mutant samples by immunohistochemistry. These results indicate that G34R-mutant tumors are likely to show extensive infiltration and alterations in global histone trimethylation might also play an important role in G34R mutant tumors.








Similar content being viewed by others
References
Schwartzentruber J, Korshunov A, Liu XY et al (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482:226–231
Wu G, Broniscer A, McEachron TA et al (2012) Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet 44:251–253
Wu G, Diaz AK, Paugh BS et al (2014) The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet 46:444–450
Fontebasso AM, Liu XY, Sturm D et al (2013) Chromatin remodeling defects in pediatric and young adult glioblastoma: a tale of a variant histone 3 tail. Brain Pathol 23:210–216
Sturm D, Bender S, Jones DT et al (2014) Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat Rev Cancer 14:92–107
Sturm D, Witt H, Hovestadt V et al (2012) Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22:425–437
Khuong-Quang DA, Buczkowicz P, Rakopoulos P et al (2012) K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol 124:439–447
Bjerke L, Mackay A, Nandhabalan M et al (2013) Histone H3.3. mutations drive pediatric glioblastoma through upregulation of MYCN. Cancer Discov 3:512–519
Pathak P, Jha P, Purkait S et al (2015) Altered global histone-trimethylation code and H3F3A-ATRX mutation in pediatric GBM. J Neurooncol 121:489–497
Bender S, Tang Y, Lindroth AM et al (2013) Reduced H3K27me3 and DNA hypomethylation are major drivers of gene expression in K27M mutant pediatric high-grade gliomas. Cancer Cell 24:660–672
Chan KM, Fang D, Gan H et al (2013) The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev 27:985–990
Lewis PW, Muller MM, Koletsky MS et al (2013) Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science 340:857–861
Venneti S, Garimella MT, Sullivan LM et al (2013) Evaluation of histone 3 lysine 27 trimethylation (H3K27me3) and enhancer of Zest 2 (EZH2) in pediatric glial and glioneuronal tumors shows decreased H3K27me3 in H3F3A K27M mutant glioblastomas. Brain Pathol 23:558–564
Venneti S, Santi M, Felicella MM et al (2014) A sensitive and specific histopathologic prognostic marker for H3F3A K27M mutant pediatric glioblastomas. Acta Neuropathol 128:743–753
Hatae R, Hata N, Yoshimoto K et al (2016) Precise detection of IDH1/2 and BRAF hotspot mutations in clinical glioma tissues by a differential calculus analysis of high-resolution melting data. PLoS One 11:e0160489
Hatae R, Hata N, Suzuki SO et al (2016) A comprehensive analysis identifies BRAF hotspot mutations associated with gliomas with peculiar epithelial morphology. Neuropathology
Neumann JE, Dorostkar MM, Korshunov A et al (2016) Distinct histomorphology in molecular subgroups of glioblastomas in young patients. J Neuropathol Exp Neurol 75:408–414
Broniscer A, Chamdine O, Hwang S et al (2016) Gliomatosis cerebri in children shares molecular characteristics with other pediatric gliomas. Acta Neuropathol 131:299–307
Herrlinger U, Jones DT, Glas M et al (2016) Gliomatosis cerebri: no evidence for a separate brain tumor entity. Acta Neuropathol 131:309–319
Lehman NL, Hattab EM, Mobley BC et al (2017) Morphological and molecular features of astroblastoma, including BRAFV600E mutations, suggest an ontological relationship to other cortical-based gliomas of children and young adults. Neuro Oncol 19:31–42
Fu YJ, Taniguchi Y, Takeuchi S et al (2013) Cerebral astroblastoma in an adult: an immunohistochemical, ultrastructural and genetic study. Neuropathology 33:312–319
Sturm D, Orr BA, Toprak UH et al (2016) New brain tumor entities emerge from molecular classification of CNS-PNETs. Cell 164:1060–1072
Gessi M, Gielen GH, Hammes J et al (2013) H3.3 G34R mutations in pediatric primitive neuroectodermal tumors of central nervous system (CNS-PNET) and pediatric glioblastomas: possible diagnostic and therapeutic implications? J Neurooncol 112:67–72
Korshunov A, Capper D, Reuss D et al (2016) Histologically distinct neuroepithelial tumors with histone 3 G34 mutation are molecularly similar and comprise a single nosologic entity. Acta Neuropathol 131:137–146
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Ebrahimi A, Skardelly M, Bonzheim I et al (2016) ATRX immunostaining predicts IDH and H3F3A status in gliomas. Acta Neuropathol Commun 4:60
Acknowledgements
This study was funded by the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant No. 26462185).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Yoshimoto, K., Hatae, R., Sangatsuda, Y. et al. Prevalence and clinicopathological features of H3.3 G34-mutant high-grade gliomas: a retrospective study of 411 consecutive glioma cases in a single institution. Brain Tumor Pathol 34, 103–112 (2017). https://doi.org/10.1007/s10014-017-0287-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10014-017-0287-7