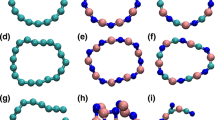
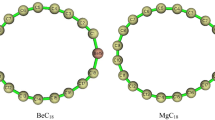
Abstract
Catenated nitrogen chains (CNCs) attract considerable attention because of their structural novelty, their high energy, and their potential as precursors for all-nitrogen materials. However, pure CNCs are unstable under ambient conditions, limiting their on-site or practical applications. Chemical riveting of a CNC involves the maintenance of the structure of a CNC and placing the CNC in a special chemical structure as a moiety of an entire molecule to stabilize it. Other structures, except from the CNC, are named rivets. In this study, the molecular geometry, bond order, natural bond orbital charge, and frontier orbital among the pure CNCs (Nx), Nx−, Nx+, and NxHy (x = 2–11) and CNCs riveted and implemented experimentally in several former studies are systematically analyzed and compared. The lone-pair repulsion between two neighboring N atoms is one of the primary factors for the low molecular stability of the pure CNCs and their derivatives. The riveted CNCs with rings are usually more stable than those with open chains. Moreover, the standard deviation of the bond orders or the bond lengths can be employed as an indicator for molecular stability, i.e., a lower standard deviation suggests higher molecular stability. Hence, electron-donating groups, groups with electronegativity within those of H and N atoms, as well as atoms or groups with empty orbitals are proposed to trap the lone pairs of CNCs as rivets to stabilize the CNCs. This strategy is expected to facilitate the creation of stable CNC-contained compounds with high structural novelty or high energy contents.

A strategy is proposed for chemically riveting instable CNCs to transform them into more stable ones.









Similar content being viewed by others
References
Dong H (2004) The development and countermeasure of high energy density materials. Chin J Energ Mater 12(Z1):1–12. http://www.energetic-materials.org.cn/hncl/ch/reader/view_abstract.aspx?doi=10.11943/j.issn.1006-9941.2015.04.014. Accessed 31 Jan 2018
Zhang CY (2018) On the energy & safety contradiction of energetic materials and the strategy for developing low-sensitive high-energetic materials. Chin J Energ Mater 26:2–10. http://www.energetic-materials.org.cn/hncl/ch/reader/view_abstract.aspx?doi=10.11943/j.issn.1006-9941.2018.01.001. Accessed 18 Jan 2018
Fried LE, Manaa MR, Pagoria PF, Simpson RL (2001) Design and synthesis of energetic materials. Annu Rev Mater Res 31:291–321. https://doi.org/10.1146/annurev.matsci.31.1.291
Li Y, Li S, Qi C, Zhang H, Zhu M, Pang S (2011) Synthesis and performance of a novel poly-nitrogen compound 1,1-azobis-1,2,3-triazole. Acta Chim Sin 69:2159–2165. http://sioc-journal.cn/Jwk_hxxb/CN/Y2011/V69/I18/2159. Accessed 24 Sept 2013
Klapötke TM, Piercey DG, Stierstorfer J (2013) The 1,3-diamino-1,2,3-triazolium cation: a highly energetic moiety. Eur J Inorg Chem 9:1509–1517. https://doi.org/10.1002/ejic.201201237
Göbel M, Karaghiosoff K, Klapötke TM, Piercey DG, Stierstorfer J (2010) Nitrotetrazolate-2N-oxides and the strategy of n-oxide introduction. J Am Chem Soc 132:17216–17226. https://doi.org/10.1021/ja106892a
Klapötke TM, Piercey DG, Stierstorfer J (2012) The 1,4,5-triaminotetrazolium cation (CN7H6 +): a highly nitrogen-rich moiety. Eur J Inorg Chem 34:5694–5700. https://doi.org/10.1002/ejic.201200964
Klapötke TM, Petermayer C, Piercey DG, Stierstorfer J (2012) 1,3-Bis(nitroimido)-1,2,3-triazolate anion, the N-nitroimide moiety, and the strategy of alternating positive and negative charges in the design of energetic materials. J Am Chem Soc 134:20827–20836. https://doi.org/10.1021/ja310384y
Tang Y, Yang H, Wu B, Ju X, Lu C, Cheng G (2013) Synthesis and characterization of a stable, catenated N11 energetic salt. Angew Chem Int Ed 52:4875–4877. https://doi.org/10.1002/anie.201300117
Li YC, Qi C, Li SH, Zhang HJ, Sun CH, Yu YZ et al (2010) 1,1’-Azobis-1,2,3-triazole: a high-nitrogen compound with stable N8 structure and photochromism. J Am Chem Soc 132:12172–12173. https://doi.org/10.1021/ja103525v
Klapötke TM, Piercey DG, Stierstorfer J (2012) Amination of energetic anions: high-performing energetic materials. Dalton Trans 41:9451–9459. https://doi.org/10.1039/C2DT30684K
Klapötke TM, Piercey DG (2011) 1,1’-Azobis(tetrazole): a highly energetic nitrogen-rich compound with a N10 chain. Inorg Chem 50:2732–2734. https://doi.org/10.1021/ic200071q
Tang Y, Yang H, Shen J, Wu B, Ju X, Lu C et al (2012) Synthesis and characterization of 1,1’-azobis(5-methyltetrazole). New J Chem 36:2447–2450. https://doi.org/10.1039/C2NJ40731K
Li SH, Pang SP, Li XT, Yu YZ, Zhao XQ (2007) Synthesis of new tetrazene(N–NN–N)-linked bi(1,2,4-triazole). Chin Chem Lett 18:1176–1178. https://doi.org/10.1016/j.cclet.2007.08.018
Li S, Shi H, Sun C, Li X, Pang S, Yu Y et al (2009) Synthesis and crystal structure of nitrogen-rich compound: 2,5,2’-triazido-1,1’-azo-1,3,4-triazole. J Chem Crystallogr 39:13–16. https://doi.org/10.1007/s10870-008-9411-1
Qi C, Li S, Li Y, Wang Y, Chen X, Pang S (2011) A novel stable high-nitrogen energetic material: 4,4’-azobis(1,2,4-triazole). J Mater Chem 21:3221–3225. https://doi.org/10.1039/C0JM02970J
Yu Q, Wang Z, Wu B, Yang H, Ju X, Lu C, Cheng G et al (2015) A Study of N-trinitroethyl-substituted aminofurazans: high detonation performance energetic compounds with good oxygen balance. J Mater Chem A 3:8156–8164. https://doi.org/10.1039/C4TA06974A
Zhang QH, Shreeve JM (2013) Growing catenated nitrogen atom chains. Angew Chem Int Ed 52:8792–8794. https://doi.org/10.1002/anie.201303297
Curtius T (1890) Ueber Stickstoffwasserstoffsäure (Azoimid) N3H. Eur J Inorg Chem 23:3023–3033. https://doi.org/10.1002/cber.189002302232
Xu Y, Wang Q, Shen C, Lin Q, Wang P, Lu M (2017) A series of energetic metal pentazolate hydrates. Nature 549:78–81. https://doi.org/10.1038/nature23662
Zhang C, Sun C, Hu B, Yu C, Lu M (2017) Synthesis and characterization of the pentazolate anion cyclo-N5 – in (N5)6(H3O)3(NH4)4Cl. Science 355:374–376. https://doi.org/10.1126/science.aah3840
Christe KO, Wilson WW, Sheehy JA, Boatz JA (1999) N5 +: a novel homoleptic polynitrogen ion as a high energy density material. Angew Chem Int Ed 38:2004–2009. https://doi.org/10.1002/(SICI)1521-3773(19990712)38:13/14<2004::AID-ANIE2004>3.0.CO;2-7
Eremet MI, Gavriliuk AG, Trojan IA, Dzivenko DA, Boehler R (2004) Single-bonded cubic form of nitrogen. Nat Mater 3:558–563. https://doi.org/10.1038/nmat1146
Eremet MI, Hemley RJ, Mao HK, Gregoryanz E (2001) Semiconducting non-molecular nitrogen up to 240 GPa and its low-pressure stability. Nature 411:170–174. https://doi.org/10.1038/35075531
Goncharov AF, Gregoryanz E, Mao HK, Liu Z, Hemley RJ (2000) Optical evidence for a nonmolecular phase of nitrogen above 150 GPa. Phys Rev Lett 85:1262–1265. https://doi.org/10.1103/PhysRevLett.85.1262
Ma Y, Oganov AR, Li Z, Xie Y, Kotakoski J (2009) Novel high pressure structures of polymeric nitrogen. Phys Rev Lett 102:065501. https://doi.org/10.1103/PhysRevLett.102.065501
Vij A, Pavlovich JG, Wilson WW, Vij V, Christe KO (2002) Experimental detection of the pentaazacyclopentadienide (pentazolate) anion, cyclo-N5 −. Angew Chem Int Ed 341:3051–3054. https://doi.org/10.1002/1521-3773(20020816)41:16<3051:AID-ANIE3051>3.0.CO;2-T
Samartzis PC, Lin JJ, Ching TT, Chaudhuri C, Lee YT, Lee SH et al (2005) Two photoionization thresholds of N3 produced by ClN3 photodissociation at 248 nm: further evidence for cyclic N3. J Chem Phys 123:051101. https://doi.org/10.1063/1.1993590
Cacace F, de Petris G, Troiani A (2002) Experimental detection of tetranitrogen. Science 295:480–481. https://doi.org/10.1126/science.1067681
Helen HH, James KO, Richard J, Van B, Svetlana BR, Mark JK (1996) Evidence for inelastic processes for N3 + and N4 + from ion energy distributions in He/N2 radio frequency glow discharges. J Appl Phys 79:93–98. https://doi.org/10.1063/1.360795
Politzer P, Murray JS (2015) Impact sensitivity and the maximum heat of detonation. J Mol Model 21:262. https://doi.org/10.1007/s00894-015-2793-z
Politzer P, Murray JS (2016) High performance, low sensitivity: conflicting or compatible? Propell Explos Pyrot 41:414–425. https://doi.org/10.1002/prep.201500349
Politzer P, Murray JS (2017) Computational analysis of polyazoles and their N-oxides. Struct Chem 28:1045–1063. https://doi.org/10.1007/s11224-016-0909-4
Moller C, Plesset MS (1934) Note on an approximation treatment for many-electron systems. Phys Rev 46:618–622. https://doi.org/10.1103/PhysRev.46.618
Dunning Jr T (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90:1007–1023. https://doi.org/10.1063/1.456153
Li Q, Liu Y (2002) Structures and stability of N11 cluster. Chem Phys Lett 353:204–212. https://doi.org/10.1016/S0009-2614(02)00018-0
Qi C, Zhang R, Pang S (2013) Thermal stability of the N10 compound with extended nitrogen chain. RSC Adv 3:17741–17748. https://doi.org/10.1039/C3RA42439A
Yin P, Li Q (2004) Structures and stability of N13 + and N13 − clusters. J Mol Model 10:13–18. https://doi.org/10.1007/s00894-003-0160-y
Thomas J, Fairman K, Strout DL (2010) Structure and decomposition energies of high-energy open-chain carbon−nitrogen compounds NxC2. J Phys Chem A 114:1144–1146. https://doi.org/10.1021/jp909489r
Casey K, Thomas J, Fairman K, Strout DL (2008) Stability and dissociation energies of open-chain N4C2. J Chem Theory Comput 4:1423–1427. https://doi.org/10.1021/ct8001943
Frisch MJ, Trucks GW, Schlegel HB et al (2004) Gaussian 03, Revision C.02. Gaussian, Inc., Wallingford
Weinhold F, Landis CR (2005) Valency and bonding: a natural bond orbital donor-acceptor perspective. Cambridge University Press, New York, p 2005
Evers J, Göbel M, Krumm B, Martin F, Medvedyev S, Oehlinger G et al (2011) Molecular structure of hydrazoic acid with hydrogen-bonded tetramers in nearly planar layers. J Am Chem Soc 133:12100–12105. https://doi.org/10.1021/ja2027053
Dixon DA, Feller D, Christe KO, Wilson WW, Vij A, Vij V et al (2004) Enthalpies of formation of gas-phase N3, N3 -, N5 +, and N5 - from ab initio molecular orbital theory, stability predictions for N5 +N3 - and N5 +N5 -, and experimental evidence for the instability of N5 +N3 -. J Am Chem Soc 126:834–843. https://doi.org/10.1021/ja0303182
Nguyen MM (2003) Polynitrogen compounds: 1. Structure and stability of N4 and N5 systems. Coord Chem Rev 244:93–113. https://doi.org/10.1016/S0010-8545(03)00101-2
Bittererová M, Brinck T (2000) Theoretical study of the triplet N4 potential energy surface. J Phys Chem A 104:11999–12005. https://doi.org/10.1021/jp002651n
Bazanov B, Geiger U, Carmieli R, Grinstein D, Welner S, Haas Y (2016) Detection of cyclo-N5 - in THF solution. Angew Chem Int Ed 55:13233–13235. https://doi.org/10.1002/anie.201605400
Hirshberg B, Gerber RB (2012) Decomposition mechanisms and dynamics of N6: bond orders and partial charges along classical trajectories. Chem Phys Lett 531:46–51. https://doi.org/10.1016/j.cplett.2012.01.088
Greschner MJ, Zhang M, Majumdar A, Liu H, Peng F, Tse JS et al (2016) A new allotrope of nitrogen as high-energy density material. J Phys Chem A 120:2920–2925. https://doi.org/10.1021/acs.jpca.6b01655
Li QS, Hu XG, Xu WG (1998) Structure and stability of N7 cluster. Chem Phys Lett 287:94–99. https://doi.org/10.1016/S0009-2614(98)00126-2
Wang, X., Ren, Y., Shuai,M., Wong, N., Li, W., Tian, A. (2001). Structure and stability of new N7 isomers. J Mol Struct THEOCHEM, 538, 145-156. https://doi.org/10.1016/S0166-1280(00)00701-6
Wang X, Tian A, Wong N, Law C, Li W (2001) A Gaussian-3 investigation of N7 isomers. Chem Phys Lett 338:367–374. https://doi.org/10.1016/S0009-2614(01)00279-2
Law C, Li W, Wang X, Tian A, Wong NB (2002) A Gaussian-3 study of N7 + and N7 − isomers. J Mol Struct THEOCHEM 617:121–131. https://doi.org/10.1016/S0166-1280(02)00411-6
Liu YD, Zhao JF, Li QS (2002) Structures and stability of N7 + and N7 − clusters. Theor Chem Accounts 107:140–146. https://doi.org/10.1007/s00214-001-0311-0
Hirshberg B, Gerber RB, Krylov AI (2014) Calculations predict a stable molecular crystal of N8. Nat Chem 6:52–56. https://doi.org/10.1038/nchem.1818
Chung G, Schmidt MW, Gordon MS (2000) An ab initio study of potential energy surfaces for N8 isomers. J Phys Chem A 104:5647–5650. https://doi.org/10.1021/jp0004361
Wu Z, Benchafia EM, Iqbal Z, Wang X (2014) N8-polynitrogen stabilized on multi-wall carbon nanotubes for oxygen-reduction reactions at ambient conditions. Angew Chem Int Ed 53:12555–12559. https://doi.org/10.1002/anie.201403060
Li QS, Wang LJ (2001) Theoretical studies on the potential energy surfaces of N8 clusters. J Phys Chem A 105:1979–1982. https://doi.org/10.1021/jp003086r
Fau S, Bartlett RJ Possible products of the end-on addition of N3 - to N5 + and their stability. J Phys Chem A 105(2001):4096–4106. https://doi.org/10.1021/jp003970h
Li QS, Wang LJ (2001) A quantum chemical theoretical study of decomposition pathways of N9 (C2v) and N9 + (C2v) clusters. J Phys Chem A 105:1203–1207. https://doi.org/10.1021/jp003461f
Thompson MD, Bledson TM, Strout DL (2002) Dissociation barriers for odd-numbered acyclic nitrogen molecules N9 and N11. J Phys Chem A 106:6880–6882. https://doi.org/10.1021/jp0208182
Wang LJ, Mezey PG, Zgierski MZ (2004) Stability and the structures of nitrogen clusters N10. Chem Phys Lett 391:338–343. https://doi.org/10.1016/j.cplett.2004.04.114
Sanderson RT (1976) Chemical bonds and bond energy. Academic Press, New York, p 1976
Sanderson RT (1988) Principles of electronegativity Part I. General nature. J Chem Educ 65:112–118. https://doi.org/10.1021/ed065p112
Sanderson RT (1988) Principles of electronegativity Part II. Applications. J Chem Educ 65:227–231. https://doi.org/10.1021/ed065p227
Christe KO, Haiges R, Wilson WW, Boatz JA (2010) Synthesis and properties of N7O+. Inorg Chem 49:1245–1251. https://doi.org/10.1021/ic9022213
Funding
This study received financial support from the National Natural Science Foundation of China (No. 11602239), the Science and Technology Fund of CAEP (2015B0302052), and Horizontal project (18zh005604, 18zh0006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information SI.
It contains bond lengths, bond orders and NBO charges of related CNC species, and molecular orbitals of some CNCs and their relatives. (DOC 2092 kb)
Rights and permissions
About this article
Cite this article
Wei, X., Wang, R. & Liu, M. Strategy for chemically riveting catenated nitrogen chains. J Mol Model 25, 345 (2019). https://doi.org/10.1007/s00894-019-4228-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4228-8