Abstract
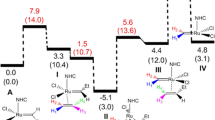
The chemical reactivity of the first- and second-generation Grubbs catalysts has always been a significant issue in olefin metathesis. In the present work, we study the [2+2] cycloreversion/cycloaddition and the alkylidene rotation involved into the interconversion of the ruthenacyclobutane intermediate, through the reaction force and reaction force constant analysis. It has been found that the structural contribution controls the barrier energy in the interconversion of ruthenacyclobutane via [2+2] cycloreversion/cycloaddition, which is slightly lower in the second generation of Grubbs catalysts while its electronic contribution is slightly higher, which unveils a major rigidity and donor/acceptor properties of the NHC. This finding explains a greater structural contribution in the rate constant. Moreover, on the basis of the reaction force constant, the process can be classified as “two-stage”-concerted reactions, noting a more asynchronous process when the first generation is used as a catalyst.
Finally, a similar analysis into the alkylidene rotation was performed. It was determined that [2+2] cycloreversion and alkylidene rotations take place in a sequential manner, the energy barrier is again controlled by structural reorganization, and the pathway is less asynchronous.








Similar content being viewed by others
References
Grubbs RH, Wenzel AG (2015) Handbook of metathesis, volume 1: catalyst development and mechanism. WILEY-VCH, Weinheim,Germany
Feng K, Xie N, Chen B et al (2016) Modular design of poly(norbornenes) for organelle-specific imaging in tumor cells. Biomacromolecules 17:538–545. https://doi.org/10.1021/acs.biomac.5b01450
Hughes DL (2016) Highlights of the recent U.S. patent literature: focus on metathesis. Org Process Res Dev 20:1008–1015. https://doi.org/10.1021/acs.oprd.6b00167
Sinclair F, Alkattan M, Prunet J, Shaver MP (2017) Olefin cross metathesis and ring-closing metathesis in polymer chemistry. Polym Chem 8:3385–3398. https://doi.org/10.1039/c7py00340d
Liu P, Ai C (2018) Olefin metathesis reaction in rubber chemistry and industry and beyond. Ind Eng Chem Res 57:3807–3820. https://doi.org/10.1021/acs.iecr.7b03830
Mcconville DH, Wolf JR, Schrock RR (1993) Synthesis of chiral molybdenum ROMP initiators and all-cis highly tactic poly(2,3-(R)2norbornadiene) (R= CF3 or CO2Me). J Am Chem Soc 115:4413–4414. https://doi.org/10.1021/ja00063a090
Schrock RR (2006) Multiple metal-carbon bonds for catalytic metathesis reactions (Nobel lecture). Angew Chem Int Ed Eng 45:3748–3759. https://doi.org/10.1002/anie.200600085
Singh R, Schrock RR, Müller P, Hoveyda AH (2007) Synthesis of monoalkoxide monopyrrolyl complexes Mo(NR)(CHR′) (OR″)(pyrrolyl): Enyne metathesis with high oxidation state catalysts. J Am Chem Soc 129:12654–12655. https://doi.org/10.1021/ja075569f
Samojłowicz C, Bieniek M, Grela K (2009) Ruthenium-based olefin metathesis catalysts bearing N-heterocyclic carbene ligands. Chem Rev 109:3708–3742. https://doi.org/10.1021/cr800524f
Vougioukalakis GC, Grubbs RH (2010) Ruthenium-based heterocyclic carbene-coordinated olefin metathesis catalysts. Chem Rev 110:1746–1787. https://doi.org/10.1021/cr9002424
Schwab P, Grubbs RH, Ziller JW, August RV (1996) Synthesis and applications of RuCl2 (=CHR′)( PR3)2 : the influence of the alkylidene moiety on metathesis activity. J Am Chem Soc 118:100–110. https://doi.org/10.1021/ja952676d
Scholl M, Ding S, Lee CW, Grubbs RH (1999) Synthesis and activity of a new generation of ruthenium-based olefin metathesis catalysts coordinated with 1,3-dimesityl-4,5-dihydroimidazol-2-ylidene ligands. Org Lett 1:953–956. https://doi.org/10.1021/ol990909q
Huang J, Stevens ED, Nolan SP et al (1999) Olefin metathesis-active ruthenium complexes bearing a nucleophilic carbene ligand. J Am Chem Soc 121:2674–2678. https://doi.org/10.1021/ja9831352
Keitz BK, Endo K, Patel PR et al (2012) Improved ruthenium catalysts for Z-selective olefin metathesis. J Am Chem Soc 134:693–699. https://doi.org/10.1021/ja210225e
Khan RKM, Torker S, Hoveyda AH (2014) Reactivity and selectivity differences between catecholate and catechothiolate ru complexes. Implications regarding design of stereoselective olefin metathesis catalysts. J Am Chem Soc 136:14337–14340. https://doi.org/10.1021/ja505961z
Herisson PJ-L, Chauvin Y (1970) Catalyse de transformation des olefines par les complexes du tugstene. Macromol Chem Phys:161–176. https://doi.org/10.1002/macp.1971.021410112
Chauvin Y (2006) Olefin metathesis: the early days (Nobel lecture). Angew Chem Int Ed Eng 45:3740–3747. https://doi.org/10.1002/anie.200601234
Cavallo L (2002) Mechanism of ruthenium-catalyzed olefin metathesis reactions from a theoretical perspective. J Am Chem Soc 124:8965–8973. https://doi.org/10.1021/ja016772s
Tsipis AC, Orpen a G, Harvey JN (2005) Substituent effects and the mechanism of alkene metathesis catalyzed by ruthenium dichloride catalysts. Dalton Trans:2849–2858. https://doi.org/10.1039/b506929g
Torker S, Merki D, Chen P (2008) Gas-phase thermochemistry of ruthenium carbene metathesis catalysts. J Am Chem Soc 130:4808–4814. https://doi.org/10.1021/ja078149z
Zhao Y, Truhlar DG (2007) Attractive noncovalent interactions in the mechanism of grubbs second-generation Ru catalysts for olefin metathesis. Org Lett 9:1967–1970. https://doi.org/10.1021/ol0705548
Paredes-Gil K, Solans-Monfort X, Rodriguez-Santiago L et al (2014) DFT study on the relative stabilities of substituted ruthenacyclobutane intermediates involved in olefin cross-metathesis reactions and their interconversion pathways. Organometallics 33:6065–6075. https://doi.org/10.1021/om500718a
Paredes-Gil K, Jaque P (2016) Theoretical characterization of first and second generation Grubbs catalysts in styrene cross-metathesis reactions: insights from conceptual DFT. Catal Sci Technol 6:755–766. https://doi.org/10.1039/c5cy00826c
Paredes-Gil K, Jaque P (2015) Initiation stage of alkene metathesis: insights from natural bond orbital and charge decomposition analyses. Chem Phys Lett 608:174–181. https://doi.org/10.1016/j.cplett.2014.11.007
Sanford MS, Love JA, Grubbs RH (2001) A versatile precursor for the synthesis of new ruthenium olefin metathesis catalysts. Organometallics:5314–5318
Sanford MS, Ulman M, Grubbs RH (2001) New insights into the mechanism of ruthenium-catalyzed olefin metathesis reactions. J Am Chem Soc 123:749–750. https://doi.org/10.1021/ja003582t
Minenkov Y, Occhipinti G, Heyndrickx W, Jensen VR (2012) The nature of the barrier to phosphane dissociation from Grubbs olefin metathesis catalysts. Eur J Inorg Chem 2012:1507–1516. https://doi.org/10.1002/ejic.201100932
Yang H-C, Huang Y-C, Lan Y-K et al (2011) Carbene rotamer switching explains the reverse trans effect in forming the Grubbs second-generation olefin metathesis catalyst. Organometallics 30:4196–4200. https://doi.org/10.1021/om200529m
Wenzel AG, Blake G, VanderVelde DG, Grubbs RH (2011) Characterization and dynamics of substituted ruthenacyclobutanes relevant to the olefin cross-metathesis reaction. J Am Chem Soc 133:6429–6439. https://doi.org/10.1021/ja2009746
Wenzel AG, Grubbs RH (2006) Ruthenium metallacycles derived from 14-electron complexes. New insights into olefin metathesis intermediates. J Am Chem Soc 128:16048–16049. https://doi.org/10.1021/ja0666598
Toro-Labbé A (1999) Characterization of chemical reactions from the profiles of energy, chemical potential, and hardness. J Phys Chem A 103:4398–4403. https://doi.org/10.1021/jp984187g
Politzer P, Toro-Labbé A, Gutiérrez-Oliva S, Murray JS (2012) Perspectives on the reaction force. In: Sabin JR BE (eds) (ed) Advances in quantum chemistry. pp 189–209
Jaque P, Toro-Labbé A, Politzer P, Geerlings P (2008) Reaction force constant and projected force constants of vibrational modes along the path of an intramolecular proton transfer reaction. Chem Phys Lett 456:135–140. https://doi.org/10.1016/j.cplett.2008.03.054
Politzer P, Murray JS, Jaque P (2013) Perspectives on the reaction force constant. J Mol Model 19:4111–4118. https://doi.org/10.1007/s00894-012-1713-8
Smith MW, Baran PS (2015) As simple as [2+2]. Science (80- ) 349:925–926. https://doi.org/10.1126/science.aac9883
Dewar MJS (1984) Multibond reactions cannot normally be synchronous. J Am Chem Soc 106:209–219. https://doi.org/10.1021/ja00313a042
Yepes D, Murray JS, Santos JC et al (2013) Fine structure in the transition region: reaction force analyses of water-assisted proton transfers. J Mol Model 19:2689–2697. https://doi.org/10.1007/s00894-012-1475-3
Yepes D, Murray JS, Politzer P, Jaque P (2012) The reaction force constant: an indicator of the synchronicity in double proton transfer reactions. Phys Chem Chem Phys 14:11125–11134. https://doi.org/10.1039/c2cp41064h
Yepes D, Donoso-Tauda O, Pérez P et al (2013) The reaction force constant as an indicator of synchronicity/nonsynchronicity in [4+2] cycloaddition processes. Phys Chem Chem Phys 15:7311–7320. https://doi.org/10.1039/c3cp44197k
Yepes D, Murray JS, Pérez P et al (2014) Complementarity of reaction force and electron localization function analyses of asynchronicity in bond formation in Diels-Alder reactions. Phys Chem Chem Phys 16:6726–6734. https://doi.org/10.1039/c3cp54766c
Murray JS, Yepes D, Jaque P, Politzer P (2015) Insights into some Diels-Alder cycloadditions via the electrostatic potential and the reaction force constant. Comput Theor Chem 1053:270–280. https://doi.org/10.1016/j.comptc.2014.08.010
Yepes D, Valenzuela J, Martínez-Araya JI et al (2019) Effect of the exchange-correlation functional on the synchronicity/nonsynchronicity in bond formation in Diels-Alder reactions: a reaction force constant analysis. Phys Chem Chem Phys 21:7412–7428. https://doi.org/10.1039/c8cp02284d
Fukui K (1981) The path of chemical reactions - the IRC approach. Acc Chem Res 14:363–368. https://doi.org/10.1021/ar00072a001
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527. https://doi.org/10.1021/j100377a021
Jaque P, Toro-Labbé A (2000) Theoretical study of the double proton transfer in the CHX-XH ⋯ CHX-XH (X = O, S) complexes. J Phys Chem A 104:995–1003. https://doi.org/10.1021/jp993016o
Martínez J, Toro-Labbé A (2009) The reaction force. A scalar property to characterize reaction mechanisms. J Math Chem 45:911–927. https://doi.org/10.1007/s10910-008-9478-0
Toro-Labbé A, Gutiérrez-Oliva S, Concha MC et al (2004) Analysis of two intramolecular proton transfer processes in terms of the reaction force. J Chem Phys 121:4570–4576. https://doi.org/10.1063/1.1777216
Gutiérrez-Oliva S, Herrera B, Toro-Labbé A, Chermette H (2005) On the mechanism of hydrogen transfer in the HSCH(O) ⇄ (S)CHOH and HSNO ⇄ SNOH reactions. J Phys Chem A 109:1748–1751. https://doi.org/10.1021/jp0452756
Herrera B, Toro-Labbé A (2007) The role of reaction force and chemical potential in characterizing the mechanism of double proton transfer in the adenine-uracil complex. J Phys Chem A 111:5921–5926. https://doi.org/10.1021/jp065951z
Jaque P, Toro-Labbe A, Geerlings P, De Proft F (2009) Theoretical study of the regioselectivity of [2 + 2] photocycloaddition reactions of acrolein with olefins. J Phys Chem A 113:332–344. https://doi.org/10.1021/jp807754f
Martínez-Araya JI, Quijada R, Toro-labbe A (2012) The mechanism of ethylene polymerization reaction catalyzed by group IVB metallocenes. A rational analysis through the use of reaction force. J Phys Chem C 116:21318–21325. https://doi.org/10.1021/jp302702h
Politzer P, Murray JS, Yepes D, Jaque P (2014) Driving and retarding forces in a chemical reaction. J Mol Model 20:2351–2357. https://doi.org/10.1007/s00894-014-2351-0
Toro-Labbé A, Gutiérrez-Oliva S, Murray JS, Politzer P (2009) The reaction force and the transition region of a reaction. J Mol Model 15:707–710. https://doi.org/10.1007/s00894-008-0431-8
Politzer P, Toro-Labbé A, Gutiérrez-Oliva S et al (2005) The reaction force: three key points along an intrinsic reaction coordinate. J Chem Sci 117:467–472. https://doi.org/10.1007/BF02708350
Rincón E, Jaque P, Toro-Labbé A (2006) Reaction force analysis of the effect of Mg(II) on the 1,3 intramolecular hydrogen transfer in thymine. J Phys Chem A 110:9478–9485. https://doi.org/10.1021/jp062870u
Burda JV, Toro-Labbé A, Gutiérrez-Oliva S et al (2007) Reaction force decomposition of activation barriers to elucidate solvent effects. J Phys Chem A 111:2455–2457. https://doi.org/10.1021/jp0709353
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL W, DJ F (2009) Gaussian 09 Rev. B.01
Becke AD (1988) Correlation energy of an inhomogeneous electron gas: a coordinate-space model. J Chem Phys 88:1053–1062. https://doi.org/10.1063/1.454274
Zhao Y, Truhlar DG (2006) A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J Chem Phys 125:194101–194118. https://doi.org/10.1063/1.2370993
Grimme S (2004) Accurate description of van der Waals complexes by density functional theory including empirical corrections. J Comput Chem 25:1463–1473. https://doi.org/10.1002/jcc.20078
Andrae D, H U, Dolg M et al (1990) Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor Chim Acta 77:123–141. https://doi.org/10.1007/BF01114537
Hehre WJ, Ditchfield R, Pople JA (1972) Self—consistent molecular orbital methods. XII. Further extensions of Gaussian—type basis sets for use in molecular orbital studies of organic molecules. J Chem Phys 56:2257–2261. https://doi.org/10.1063/1.1677527
Jacobsen H, Correa A, Poater A et al (2009) Understanding the M(NHC) (NHC=N-heterocyclic carbene) bond. Coord Chem Rev 253:687–703. https://doi.org/10.1016/j.ccr.2008.06.006
Antonova NS, Carbó JJ, Poblet JM (2009) Quantifying the donor−acceptor properties of phosphine and N-heterocyclic carbene ligands in Grubbs’ catalysts using a modified EDA procedure based on orbital deletion. Organometallics 28:4283–4287. https://doi.org/10.1021/om900180m
Acknowledgments
K.P-G., F.M, and P.J thank CONICYT through the FONDECYT projects 3170117, 1180158, and 1181914, respectively. Moreover, K.P-G. acknowledges “CONICYT+PAI+CONVOCATORIA NACIONAL SUBVENCIÓN A INSTALACIÓN EN LA ACADEMIA CONVOCATORIA AÑO 2018 + PAI77180024.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to Topical Collection QUITEL 2018 (44th Congress of Theoretical Chemists of Latin Expression)
Electronic supplementary material
ESM 1
Supplementary information including the Cartesian coordinates of all computed species: i) alkene coordination with 14e- inactive and olefin to the first-generation Grubbs catalysts; ii) key points along the reaction pathways to 1st-Ru-[2+2] cycloaddition; iii) key points along the reaction pathways to 2nd-Ru-[2+2] cycloaddition; and iv) key points along the reaction pathways to the alkylidene rotation in the second-generation Grubbs catalysts. (PDF 592 kb)
Rights and permissions
About this article
Cite this article
Paredes-Gil, K., Mendizábal, F. & Jaque, P. Further understanding of the Ru-centered [2+2] cycloreversion/cycloaddition involved into the interconversion of ruthenacyclobutane using the Grubbs catalysts from a reaction force analysis. J Mol Model 25, 305 (2019). https://doi.org/10.1007/s00894-019-4150-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4150-0