Abstract
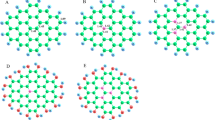
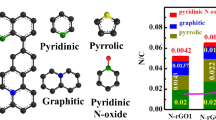
Nitrogen (N)-doped carbons reportedly exhibit good electrocatalytic activity for the oxygen reduction reaction (ORR) of fuel cells. This work provides theoretical insights into the ORR mechanism of N-doped graphene by using density functional theory calculations. All possible reaction pathways were investigated, and the transition state of each elementary step was identified. The results showed that OOH reduction was easier than O–OH breaking. OOH reduction followed a direct Eley–Rideal mechanism (the OOH species was in gas phase, but H was chemisorbed on the surface) with a significantly low reaction barrier of 0.09 eV. Pathways for both four-electron and two-electron reductions were possible. The rate-determining step of the two-electron pathway was the reduction of O2 (formation of OOH), whereas that of the four-electron pathway was the reduction of OH into H2O. After comparing the barriers of the rate-determining steps of the two pathways, we found that the two-electron pathway was more energetically favored than the four-electron pathway.





Similar content being viewed by others
References
Xiong W, Du F, Liu Y, Perez A, Supp M, Ramakrishnan TS, Dai L, Jiang L (2010) J Am Chem Soc 132:15839–15841
Chen Z, Higgins D, Tao H, Hsu RS, Chen Z (2009) J Phys Chem C 113:21008–21013
Steele BC, Heinzel A (2001) Nature 414:345–352
Winter M, Brodd RJ (2004) Chem Rev 104:4245–4270
Gasteiger HA, Kocha SS, Sompalli B, Wagner FT (2005) Appl Catal B 56:9–35
Yu X, Ye S (2007) J Power Sources 172:145–154
Gong KP, Du F, Xia ZH, Durstock M, Dai LM (2009) Science 323:760–764
Qu L, Liu Y, Baek J-BDai L (2010) ACS Nano 4:1321–1326
Geng DS, Chen Y, Chen YG, Li YL, Li RY, Sun XL, Ye SY, Knights S (2011) Energy Environ Sci 4:760–764
Kundu S, Nagaiah TC, Xia W, Wang YM, Van Dommele S, Bitter JH, Santa M, Grundmeier G, Bron M, Schuhmann W, Muhler M (2009) J Phys Chem C 113:14302–14310
Niwa H, Kobayashi M, Horiba K, Harada Y, Oshima M, Terakura K, Ikeda T, Koshigoe Y, Ozaki J-i, Miyata S, Ueda S, Yamashita Y, Yoshikawa H, Kobayashi K (2011) J Power Sources 196:1006–1011
Niwa H, Horiba K, Harada Y, Oshima M, Ikeda T, Terakura K, Ozaki J-iMiyata S (2009) J Power Sources 187:93–97
Tang YF, Allen BL, Kauffman DR, Star A (2009) J Am Chem Soc 131:13200–13201
Wang Y, Shao YY, Matson DW, Li JH, Lin YH (2010) ACS Nano 4:1790–1798
Vanin M, Gath J, Thygesen KS, Jacobsen KW (2010) Phys Rev B 82:195411 doi: 10.1103/PhysRevB.82.195411
Wang Z, Jia R, Zheng J, Zhao J, Li L, Song J, Zhu Z (2011) ACS Nano 5:1677–1684
Ma G, Jia R, Zhao J, Wang Z, Song C, Jia S, Zhu Z (2011) J Phys Chem C 115:25148–25154
Matter PH, Ozkan US (2006) Catal Lett 109:115–123
Maldonado S, Stevenson KJ (2005) J Phys Chem B 109:4707–4716
Chen S, Bi J, Zhao Y, Yang L, Zhang C, Ma Y, Wu Q, Wang XHZ (2012) Adv Mater 24:5593–5597
Zhang S, Zhang H, Liu Q, Chen S (2013) J Mater Chem A 1:3302–3308
Deng DH, Pan XL, Yu LA, Cui Y, Jiang YP, Qi J, Li WX, Fu QA, Ma XC, Xue QK, Sun GQ, Bao XH (2011) Chem Mater 23:1188–1193
Lee KR, Lee KU, Lee JW, Ahn BT, Woo SI (2010) Electrochem Commun 12:1052–1055
Huang S-F, Terakura K, Ozaki T, Ikeda T, Boero M, Oshima M, Ozaki J-iMiyata S (2009) Phys Rev B 80:235410
Kim H, Lee K, Woo SI, Jung Y (2011) Phys Chem Chem Phys 13:17505–17510
Zhang L, Xia Z (2011) J Phys Chem C 115:11170–11176
Ikeda T, Boero M, Huang S-F, Terakura K, Oshima M, Ozaki J-I (2008) J Phys Chem C 112:14706–14709
Sidik RA, Anderson AB, Subramanian NP, Kumaraguru SP, Popov BN (2006) J Phys Chem B 110:1787–1793
Lai L, Potts JR, Zhan D, Wang L, Poh CK, Tang C, Gong H, Shen Z, Lin J, Ruoff RS (2012) Energy Environ Sci 5:7936–7942
Xu Z, Li H, Fu M, Luo H, Sun H, Zhang L, Li K, Wei B, Lu J, Zhao X (2012) J Mater Chem 22:18230–18236
Okamoto Y (2009) Appl Surf Sci 256:335–341
Luo Z, Lim S, Tian Z, Shang J, Lai L, MacDonald B, Fu C, Shen Z, Yu T, Lin J (2011) J Mater Chem 21:8038–8044
Yu L, Pan X, Cao X, Hu P, Bao X (2011) J Catal 282:183–190
Payne MC, Teter MP, Allan DC, Arias T, Joannopoulos J (1992) Rev Mod Phys 64:1045–1097
Milman V, Winkler B, White J, Pickard C, Payne M, Akhmatskaya E, Nobes R (2000) Int J Quantum Chem 77:895–910
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865
Perdew JP, Chevary J, Vosko S, Jackson KA, Pederson MR, Singh D, Fiolhais C (1992) Phys Rev B 46:6671
Ge Q, Jenkins S, King D (2000) Chem Phys Lett 327:125–130
Dai JY, Yuan JM (2010) Phys Rev B 81:165414
Zhao L, He R, Rim KT, Schiros T, Kim KS, Zhou H, Gutiérrez C, Chockalingam S, Arguello CJ, Pálová L (2011) Science 333:999–1003
Wang Y, Balbuena PB (2005) J Phys Chem B 109:14896–14907
Rossmeisl J, Qu Z-W, Zhu H, Kroes G-JNørskov JK (2007) J Electroanal Chem 607:83–89
Hyman MP, Medlin JW (2006) J Phys Chem B 110:15338–15344
Zhang J, Vukmirovic MB, Xu Y, Mavrikakis M, Adzic RR (2005) Angew Chem Int Ed 44:2132–2135
Damjanovic A, Brusic V (1967) Electrochim Acta 12:615–628
Jacob T, Goddard WA (2006) Chem Phys Chem 7:992–1005
Nilekar AU, Mavrikakis M (2008) Surf Sci 602:L89–L94
Tripković V, Skúlason E, Siahrostami S, Nørskov JK, Rossmeisl J (2010) Electrochim Acta 55:7975–7981
Wang J, Markovic N, Adzic R (2004) J Phys Chem B 108:4127–4133
Studt F (2013) Catal Lett 143:58–60
Eley D, Rideal E (1940) Nature 146:401–402
Kuipers E, Vardi A, Danon A, Amirav A (1991) Phys Rev Lett 66:116
Meijer AJHM, Farebrother AJ, Clary DC, Fisher AJ (2001) J Phys Chem A 105:2173–2182
Acknowledgments
The calculations were performed at the Shanghai Supercomputing Center. This work was supported by the Natural Science Foundation of China (Nos. 20673135 and 50702065), Shanxi Natural Science Foundation (2008021029-1), and Knowledge Innovation Project of Chinese Academy of Science (No. KJCX2.YW.M10).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Wang, Z. & Zhu, Z. A density functional theory study on oxygen reduction reaction on nitrogen-doped graphene. J Mol Model 19, 5515–5521 (2013). https://doi.org/10.1007/s00894-013-2047-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-013-2047-x