Abstract
Introduction
Eldecalcitol increases bone mineral density (BMD) and reduces vertebral fracture in patients with primary osteoporosis. However, the effect of eldecalcitol on BMD and fracture in glucocorticoid-induced osteoporosis (GIO) patients is unknown. This study was undertaken to compare the effect of eldecalcitol on BMD and fracture with that of alfacalcidol in GIO patients.
Materials and methods
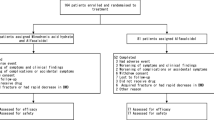
A randomized, open-label, parallel group study was conducted to identify the effectiveness and safety of monotherapy with 0.75 μg eldecalcitol compared with 1.0 μg alfacalcidol in GIO patients.
Results
Lumbar spine BMD increased with eldecalcitol, but decreased with alfacalcidol at 12 and 24 months (between group difference 1.29%, p < 0.01, and 1.10%, p < 0.05, respectively). Total hip and femoral neck BMD were maintained until 24 months by eldecalcitol, but decreased by alfacalcidol (between group difference 0.97%, p < 0.05 and 1.22%, p < 0.05, respectively). Both bone formation and resorption markers were more strongly suppressed by eldecalcitol than by alfacalcidol. Eldecalcitol showed better effect on BMD than alfacalcidol in patients with no prevalent fracture and BMD > 70% of the young adult mean, and with ≤ 3 months of previous glucocorticoid treatment. No significant difference in the incidence of vertebral fracture was found, and the incidence of adverse events was similar between the two groups.
Conclusions
Eldecalcitol was more effective than alfacalcidol in maintaining BMD in GIO patients. Because eldecalcitol was effective in patients with no or short-term previous glucocorticoid treatment, as well as those without prevalent fracture or low BMD, eldecalcitol can be a good candidate for primary prevention of GIO.
Clinical trial registration number
UMIN000011700.




Similar content being viewed by others
References
Lane NE, Lukert B (1998) The science and therapy of glucocorticoid-induced bone loss. Endocrinol Metab Clin North Am 27:465–483
Clowes JA, Peel N, Eastell R (2001) Glucocorticoid-induced osteoporosis. Curr Opin Rheumatol 13:326–332
van Staa TP, Leufkens HG, Cooper C (2002) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 13:777–787
Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C (2000) Use of oral corticosteroids and risk of fractures. J Bone Miner Res 15:993–1000
Suzuki Y, Nawata H, Soen S, Fujiwara S, Nakayama H, Tanaka I, Ozono K, Sagawa A, Takayanagi R, Tanaka H, Miki T, Masunari N, Tanaka Y (2014) Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research: 2014 update. J Bone Miner Metab 32:337–350
Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, Thamsborg G, Liberman UA, Delmas PD, Malice MP, Czachur M, Daifotis AG (1998) Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med 339:292–299
Reid DM, Hughes RA, Laan RF, Sacco-Gibson NA, Wenderoth DH, Adami S, Eusebio RA, Devogelaer JP (2000) Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Corticosteroid-Induced Osteoporosis Treatment Study. J Bone Miner Res 15:1006–1013
Reid DM, Devogelaer JP, Saag K, Roux C, Lau CS, Reginster JY, Papanastasiou P, Ferreira A, Hartl F, Fashola T, Mesenbrink P, Sambrook PN (2009) Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet (London, England) 373:1253–1263
Adachi JD, Saag KG, Delmas PD, Liberman UA, Emkey RD, Seeman E, Lane NE, Kaufman JM, Poubelle PE, Hawkins F, Correa-Rotter R, Menkes CJ, Rodriguez-Portales JA, Schnitzer TJ, Block JA, Wing J, McIlwain HH, Westhovens R, Brown J et al (2001) Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum 44:202–211
Wallach S, Cohen S, Reid DM, Hughes RA, Hosking DJ, Laan RF, Doherty SM, Maricic M, Rosen C, Brown J, Barton I, Chines AA (2000) Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif Tissue Int 67:277–285
Saag KG, Wagman RB, Geusens P, Adachi JD, Messina OD, Emkey R, Chapurlat R, Wang A, Pannacciulli N, Lems WF (2018) Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol 6:445–454
Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M (2007) Glucocorticoid-induced osteoporosis: a systematic review and cost-utility analysis. Health technology assessment (Winchester, England) 11:1–231
Ringe JD, Coster A, Meng T, Schacht E, Umbach R (1999) Treatment of glucocorticoid-induced osteoporosis with alfacalcidol/calcium versus vitamin D/calcium. Calcif Tissue Int 65:337–340
Sambrook PN, Kotowicz M, Nash P, Styles CB, Naganathan V, Henderson-Briffa KN, Eisman JA, Nicholson GC (2003) Prevention and treatment of glucocorticoid-induced osteoporosis: a comparison of calcitriol, vitamin D plus calcium, and alendronate plus calcium. J Bone Miner Res 18:919–924
Matsumoto T, Miki T, Hagino H, Sugimoto T, Okamoto S, Hirota T, Tanigawara Y, Hayashi Y, Fukunaga M, Shiraki M, Nakamura T (2005) A new active vitamin D, ED-71, increases bone mass in osteoporotic patients under vitamin D supplementation: a randomized, double-blind, placebo-controlled clinical trial. J Clin Endocrinol Metab 90:5031–5036
Jiang Y, Tang H, Ma X, Cheng Q, Lin H, Jin X, Zhang Z, Yu W, He S, Kobayashi T, Uehara S, Matsumoto T, Xia W (2019) Eldecalcitol increases bone mineral density in Chinese osteoporotic patients without vitamin D or calcium supplementation. J Bone Miner Metab 37:1036–1047
Matsumoto T, Ito M, Hayashi Y, Hirota T, Tanigawara Y, Sone T, Fukunaga M, Shiraki M, Nakamura T (2011) A new active vitamin D(3) analog, eldecalcitol, prevents the risk of osteoporotic fractures—a randomized, active comparator, double-blind study. Bone 49:605–612
Tamaki J, Iki M, Sato Y, Kajita E, Nishino H, Akiba T, Matsumoto T, Kagamimori S (2017) Total 25-hydroxyvitamin D levels predict fracture risk: results from the 15-year follow-up of the Japanese Population-based Osteoporosis (JPOS) Cohort Study. Osteoporos Int 28:1903–1913
Leslie WD, Martineau P, Bryanton M, Lix LM (2019) Which is the preferred site for bone mineral density monitoring as an indicator of treatment-related anti-fracture effect in routine clinical practice? A registry-based cohort study. Osteoporos Int 30:1445–1453
Acknowledgements
This study was supported by a fund from Chugai Pharmaceutical Co. Ltd.
We thank all investigators and participating patients involved in this study at the following clinical sites in Japan: Asahi General Hospital, Chiba; Chibaken Saiseikai Narashino Hospital, Chiba; Fukuoka Tokushukai Hospital, Fukuoka; Fukuoka Yutaka Central Hospital, Fukuoka; Hiroshima Red Cross Hospital and Atomic-bomb Survivors Hospital, Hiroshima; Hyogo Prefectural Nishinomiya Hospital, Hyogo; Ishikawa Prefectural Central Hospital, Ishikawa; Itami City Hospital, Hyogo; Kagawa Prefectural Central Hospital, Kagawa; Kang Rheumatology Orthopedics Clinic, Kanagawa; Kansai Electric Power Hospital, Osaka; Kansai Medical University Hospital, Osaka; Kanzaki Municipal General Hospital, Himeji; Kashimoto Hospital, Osaka; Kawakita General Hospital, Tokyo; Kawasaki Municipal Hospital, Kanagawa; Keio University Hospital, Tokyo; Kindai University Nara Hospital, Nara; Kitasato University Medical Center, Saitama; Kobe City Medical Center West Hospital, Kobe; Kyushu Medical Center, Fukuoka; Kyushu University Beppu Hospital, Oita; Kyushu University Hospital, Fukuoka; Matsuyama Red Cross Hospital, Ehime; Mie Rheumatology Clinic, Mie; Minami-Akita Orthopedics Clinic, Akita; Minami-Okayama Medical Center, Okayama; Mitsui Memorial Hospital, Tokyo; Nagoya Rheumatology Clinic, Aichi; Nakama Municipal Hospital, Fukuoka; Nakamura Orthopedics Clinic, Kagoshima; Obase Hospital, Fukuoka; Okayama saiseikai General Hospital, Okayama; Osaka City University Hospital, Osaka; Osaka Kaisei Hospital, Osaka; Osaka-Minami Medical Center, Osaka; Osaki Citizen Hospital, Miyagi; Red Cross Nagasaki Genbaku Hospital, Nagasaki; Red Cross Okayama Hospital, Okayama; Rinku General Medical Center, Osaka; Sagawa Akira Rheumatology Clinic, Hokkaido; Saiseikai Suita Hospital, Osaka; Saitama Medical Center, Saitama; Saitama Medical University Hospital, Saitama; Shiminnomori Hospital, Miyazaki; Shimokitazawa Hospital, Tokyo; Shinko Hospital, Hyogo; Shinsuma Hospital, Osaka; Shirakawa Kosei General Hospital, Fukushima; Showa University Northern Yokohama Hospital, Kanagawa; Soshigaya Okura Clinic, Tokyo; St. Marianna University School of Medicine, Kanagawa; Tohoku University Hospital, Miyagi; Tokyo Metropolitan Ohtsuka hospital, Tokyo; Tomishiro Central Hospital, Okinawa; Tonan Hospital, Hokkaido; Tsurukami Clinic of Orthopedics and Rheumatology, Kumamoto;Utazu Hospital, Kagawa; Yokosuka Kyosai Hospital, Kanagawa;Yonezawa City Hospital, Yamagata; Yoshitama Rheumatology Internal Medicine Clinic, Kagoshima; Yu-Family Clinic, Miyagi
We thank the late Chikuma Hamada, Ph.D., Department of Business Engineering, Faculty of Engineering, Tokyo University of Science, for serving as statistical consultant. We also thank Mebix Inc., Tokyo, Japan, for supporting statistical analysis and data management, monitoring, auditing, and offering assistance for writing the manuscript, Musashi Image Joho Co., Ltd., Tokyo, Japan, for supporting fracture assessment, and LSI Medience Corporation, Tokyo, Japan, for measuring bone turnover markers.
Author information
Authors and Affiliations
Contributions
TM, SS, KY, TT, YT, and ST planned the study design and performed the study. TN and MI analyzed the X-ray and adjudicated the vertebral fractures. TT analyzed the DXA and adjudicated BMD. AH planned and performed the statistical analysis. TM and SS interpreted the results. TM analyzed the results and wrote the initial draft. All authors contributed to discuss the results and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
T.M. has received consulting fees from Chugai, Amgen Astellas Biopharma, Teijin Pharma, Daiichi-Sankyo and Asahi Kasei Pharma. K.Y. has received speaking fees, and/or honoraria from AbbVie, Astellas, AYUMI Pharma, Bristol-Myers Squibb, Chugai, Eisai, Janssen, Mitsubishi-Tanabe, Ono and UCB Japan. T.T. has received grants from Astellas, Chugai, Daiichi-Sankyo, Takeda, AbbVie, Asahi Kasei Pharma, Mitsubishi-Tanabe, Pfizer, Eisai, AYUMI Pharma, Nipponkayaku, Novartis and Shionogi. Honoraria from Astellas, AbbVie, AYUMI Pharma, Eisai., Gilead Sciences, GlaxoSmithKline, Sanofi., Taiho, Mitsubishi-Tanabe, Diaichi-Sankyo, Chugai, Taisho Pharma, Eli Lilly Japan, Novartis, Boehringer-Ingelheim, Nipponkayaku, Pfizer, Bristol–Myers Squibb, Janssen and UCB Japan. Y.T. has received speaker fee and/or honoraria from Daiichi-Sankyo, Astellas, Chugai, Eli Lilly Japan, Pfizer, AbbVie, YL Biologics, Bristol-Myers Squibb, Takeda, Mitsubishi-Tanabe, Novartis, Eisai, Janssen, Teijin Pharma, and has received research grants from Asahi Kasei Pharma, Mitsubishi-Tanabe, Chugai, Takeda, Sanofi, Bristol-Myers Squibb, UCB Japan, Daiichi-Sankyo, Eisai and Ono. S.T. has received consulting fees, speaker fees, and/or honoraria from Amgen Astellas Biopharma, Astellas, Asahi Kasei Pharma, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly Japan, Ono, Pfizer, Taisho Toyama Pharma and Teijin Pharma. M.I. has received consulting fees, speaker fees, honoraria, and/or research funding from Chugai, Astellas, Asahi Kasei Pharma, Daiichi-Sankyo, Eli Lilly Japan, MSD, Ono and Pfizer. A.H. has received consulting fees, speaker fees, honoraria, and/or research funding from Astellas, Ono and Teijin Pharma. S.S. has received consulting fees, speaker fees, and/or honoraria from Asahi Kasei Pharma, Astellas, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly Japan, MSD, Ono, Pfizer, Takeda and Teijin Pharma. Other authors reported no conflict of interest related to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Matsumoto, T., Yamamoto, K., Takeuchi, T. et al. Eldecalcitol is superior to alfacalcidol in maintaining bone mineral density in glucocorticoid-induced osteoporosis patients (e-GLORIA). J Bone Miner Metab 38, 522–532 (2020). https://doi.org/10.1007/s00774-020-01091-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-020-01091-4