Abstract
Introduction
This study compared the clinical usefulness of minodronate (50 mg/4 weeks) plus alfacalcidol (1 μg/day) (Group M) with that of alfacalcidol alone (1 μg/day) (Group A) for treating glucocorticoid-induced osteoporosis.
Materials and methods
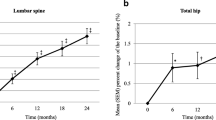
The primary endpoints were the changes from baseline in lumbar spine (LS) bone mineral density (BMD) and the cumulative incidence of vertebral fracture at 24 months; secondary endpoints included the changes from baseline in total hip (TH) BMD and bone turnover markers.
Results
Of 164 patients enrolled, 152 (Group M, n = 75; Group A, n = 77) were included in the analysis of efficacy. At each time point and at 24 months, LS BMD and TH BMD were significantly higher in Group M than in Group A. The 152 patients were divided into two subgroups that were previously treated with glucocorticoids for ≤ 3 months or > 3 months. In both subgroups, the changes from baseline in LS BMD and TH BMD from baseline at 24 months had increased more in Group M than in Group A. There were no differences found in the incidence of vertebral fracture between the groups, because the number of enrolled patients was lesser than that initially expected. In Group M, both bone formation and resorption markers significantly decreased from baseline at 3 months and maintained at 6, 12, and 24 months.
Conclusions
Minodronate plus alfacalcidol was more effective than alfacalcidol alone in increasing BMD and was effective in increasing BMD for both prevention and treatment. Therefore, minodronate can be a good candidate drug for the treatment of glucocorticoid-induced osteoporosis.





Similar content being viewed by others
References
Lane NE, Lukert B (1998) The science and therapy of glucocorticoid-induced bone loss. Endocrinol Metab Clin North Am 27:465–483
Clowes JA, Peel N, Eastell R (2001) Glucocorticoid-induced osteoporosis. Curr Opin Rheumatol 13:326–332
Van Staa TP, Leufkens HG, Cooper C (2002) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 13:777–787
Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C (2000) Use of oral corticosteroids and risk of fractures. J Bone Miner Res 15:993–1000
Nawata H, Soen S, Takayanagi R, Tanaka I, Takaoka K, Fukunaga M, Matsumoto T, Suzuki Y, Tanaka H, Fujiwara S, Miki T, Sagawa A, Nishizawa Y, Seino Y (2005) Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research (2004). J Bone Miner Metab 23:105–109
Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, Thamsborg G, Liberman UA, Delmas PD, Malice MP, Czachur M, Daifotis AG (1998) Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N Eng J Med 339:292–299
Adachi JD, Saag KG, Delmas PD, Liberman UA, Emkey RD et al (2001) Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids. Arthritis Rheum 44:202–211
Cohen S, Levy RM, Kellaer M, Boling E, Emkey RD, Greenwald M, Zizic TM, Wallach S, Sewell KL, Lukert BP, Axelrod DW, Chines AA (1999) Risedronate therapy prevents cortirosteroid-induced bone loss. Arthritis Rheum 42:2309–2318
Reid DM, Hughes RA, Laan RF, Sacco-Gibson NA, Wenderoth DH, Adami S, Eusebio RA, Devogelaer JP (2000) Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. J Bone MIner Res 15:1006–1013
Wallach S, Cohen S, Reid DM, Hughes RA, Hosking DJ, Laan RF, Doherty SM, Rosen C, Brown J, Barton I, Chines AA (2000) Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif Tissue Int 67:277–285
De Nijs RN, Jacobs JW, Algra A, Lems WF, Bijlsma JW (2004) Prevention and treatment of glucocorticoid-induced osteoporosis with active vitamin D(3) analogues: a review with meta-analysis of randomized controlled trials including organ transplantation studies. Osteoporos Int 15:589–602
Matsumoto T, Hagino H, Shiraki M, Fukunaga M, Nakano T, Takaoka K, Morii H, Ohashi Y, Nakamura T (2009) Effect of daily oral minodronate on vertebral fractures in Japanese postmenopausal women with established osteoporosis: a randomized placebo-controlled double-blind study. Osteoporos Int 20:1429–1437
Matsumoto T, Yamamoto K, Takeuchi T, Tanaka Y, Tanaka S, Nakano T, Ito M, Tomomitsu T, Hirakawa A, Soen S Eldecalcitol is more effective than alfacalcidol in increasing bone mineral density in patients with glucocorticoid-induced osteoporosis: a multicenter, randomized, open-label study (e-GLORIA). J Bone Miner Metab (Submitted)
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Metab 8:1137–1148
Mori S, Soen S, Hagino H, Nakano T, Ito M, Fujiwara S, Kato Y, Tokuhashi Y, Togawa D, Endo N, Sawaguchi T (2013) Committee for vertebral fracture evaluation. Justification criteria for vertebral fractures: year 2012 revision. J Bone Miner Metab 31:258–261
Ringe JD, Dorst A, Faber H, Ibach K, Sorenson F (2003) Intermittent intravenous ibandronate injections reduce vertebral fracture risk in corticosteroid-induced osteoporosis: results from a long-term comparative study. Osteoporos Int 14:801–807
Suzuki Y, Nawata H, Soen S, Fujiwara S, Nakayama H, Tanaka I, Ozono K, Sagawa A, Takayanagi R, Tanaka H, Miki T, Masunari N, Tanaka Y (2014) Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research: 2014 update. J Bone Miner Metab 32:337–350
Craig H, Mallinckrod, Peter W, Lane, Dan Schnell, Yahong Peng, James P. Mancuso (2008) Recommendations for the Primary analysis of continuous endpoints in longitudinal clinical trials. Therapeutic Innovation Regulatory Science https://doi.org/10.1177/009286150804200402
Okazaki R, Hagino H, Ito M, Sone T, Nakamura T, Mizunuma H, Fukunaga M, Shiraki M, Nishizawa Y, Ohashi Y, Matsumoto T (2012) Efficacy and safety of monthly oral minodoronate in patients with involutional osteoporosis. Osteoporos Int 23:1737–1745
Matsumoto T, Ito M, Hayashi Y, Hirota T, Tanigawara Y, Sone T, Fukunaga M, Shiraki M, Nakamura T (2011) A new active vitaminD3 analog, eldecalcitol, prevents the risk of osteoporotic fractures- A randomized, active comparator, double-blind study. Bone 49:605–612
Director, Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare. PFSB/ELD Notification No.0707–1 (July 7, 2017) Guideline for Clinical Evaluation of Osteoporosis Agents (revised edition)
Hagino H, Shiraki M, Fukunaga M, Nakano T, Takaoka K, Ohashi Y, Nakamura T, Matsumoto T (2012) Three years of treatment with minodoronate in patients with postmenopausal osteoporosis. J Bone Miner Metab 30:439–446
Task Force Committee of bone metabolic markers, Japan Osteoporosis Society (2018) Guidelines for the use of bone metabolic markers in the diagnosis and treatment of osteoporosis, 2018th edn. Japan Osteoporosis Society, Tokyo
Acknowledgement
We thank all investigators and participating patients involved in this study at the following clinical sites in Japan: Yokohama Rosai Hospital, Murayama Medical Center,Katayama Seikeigeka Rheumatism Clinic, Toyota Kosei Hospital, Kouseiren Namerikawa Hospital, Suzuki-clinic, Clinic of a wind, Komazawa, Saitama Medical Center, Juntendo University Shizuoka Hospital, Kanazawa Medical University Himi Municipal Hospital, JCHO Chukyo Hospital, Japanese Red Cross Medical Center, Shirasawa Orthopedic Clinic, Saito Clinic, Takikawa Municipal Hospital, NHO Asahikawa Medical Center, Soshigaya Okura Clinic, Hokkaido Medical Center for Rheumatic Diseases, Kawasaki Municipal Hospital, Tohno Chuo Clinic, Tokyo Dental College Ichikawa General Hospital, Tokushima University Hospital, Kindai University Nara Hospital, Sagawa Akira Rheumatology Clinic, Utazu Hoapital, Saiseikai Kanazawa Hospital, Sasebo Chuo Hospital, Tomishiro Central Hospital, NHO Kure Medical Center and Chugoku Cancer Center, Matsuyama Red Cross Hospital, Matsubara Rheumatology and Orthopedics Clinic, Kansai Medical University Hospital, MAYFLOWER Hospital, Shiminnomori Hospital, Kumamoto Kinoh Hospital, Tsurukami Clinic of Orthopedics and Rheumatology, Japanese Red Cross Okayama Hospital, Tokushima National Hospital, Harada Hospital, Yodogawa Christian Hospital, Okayama Saiseikai General Hospital, Higashiosaka City Medical Center, Minamiosaka Hospital. We thank the late Ph.D. Chikuma Hamada, Department of Business Engineering, Faculty of Engineering, Tokyo University of Science, for his statistical consultant. In addition, we thank Linical Co., Ltd.(Osaka, Japan), which supported the executive office, monitoring, audit, and writing assistance for this manuscript, Meditrix Corporation (Tokyo, Japan), which supported data management, SOC Co., Ltd. (Tokyo, Japan), which supported statistical analysis, MICRON Inc. (Tokyo, Japan) which supported the decision office for bone fractures and bone mineral density, and LSI Medience Corporation (Tokyo, Japan), which supported the measurement of bone turnover markers.
Funding
This study was funded by Astellas Pharma Inc. and Ono pharmaceutical Co., LTD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S.S. has received consulting fees, speaking fees, and/or honoraria from Asahi Kasei Pharma, Astellas, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly Japan, MSD, Ono, Pfizer, Takeda and Teijin Pharma. K.Y. has received speaking fees, and/or honoraria from AbbVie, Astellas, AYUMI Pharma, Bristol-Myers Squibb, Chugai, Eisai, Janssen, Mitsubishi-Tanabe, Ono and UCB Japan. T.T. has received grants from Astellas, Chugai, Daiichi-Sankyo, Takeda, AbbVie, Asahi kasei Pharma., Mitsubishi-Tanabe, Pfizer, Eisai, AYUMI Pharma, Nipponkayaku., Novartis and Shionogi. Personal Fee from: Astellas, AbbVie, AYUMI Pharma, Eisai., Gilead Sciences, GlaxoSmithKline, Sanofi., Taiho, Mitsubishi-Tanabe, Diaichi-Sankyo, Chugai, Taisho Pharma, Eli Lilly Japan, Novartis, Boehringer-ingelheim, Nipponkayaku, Pfizer、Bristol–Myers Squibb, Janssen and UCB Japan. Y.T. has received speaking fees and/or honoraria from Daiichi-Sankyo, Astellas, Chugai, Eli Lilly Japan, Pfizer, AbbVie, YL Biologics, Bristol-Myers Squibb, Takeda, Mitsubishi-Tanabe, Novartis, Eisai, Janssen, Teijin Pharma and has received research grants from Asahi kasei Pharma, Mitsubishi-Tanabe, Chugai, Takeda, Sanofi, Bristol-Myers Squibb, UCB Japan, Daiichi-Sankyo, Eisai and Ono. S.T. has received consulting fees, speaking fees, and/or honoraria from Amgen Astellas Biopharma, Astellas, Asahi Kasei Pharma, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly Japan, Ono, Pfizer, Taisho Toyama Pharma and Teijin Pharma. M.I. has received consulting fees, speaking fees, honoraria, and/or research funding from Chugai, Astellas, Asahi Kasei Pharma, Daiichi-Sankyo, Eli Lilly Japan, MSD, Ono and Pfizer. H.H. has received lecture fees or grants from Asahi Kasei Pharma, Astellas, Chugai, Eisai, Eli Lilly Japan, Mitsubishi -Tanabe, Mochida, Ono, Pfizer, Taisho Toyama, Takeda, Teijin Pharma, MSD and UCB. A.H. has received consulting fees, speaking fees, honoraria, and/or research funding from Astellas, Ono and Teijin Pharma. T.M. has received consulting fees from Chugai, Amgen Astellas Biopharma, Teijin Pharma, Daiichi-Sankyo and Asahi Kasei Pharma.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Soen, S., Yamamoto, K., Takeuchi, T. et al. Minodronate combined with alfacalcidol versus alfacalcidol alone for glucocorticoid-induced osteoporosis: a multicenter, randomized, comparative study. J Bone Miner Metab 38, 511–521 (2020). https://doi.org/10.1007/s00774-019-01077-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-019-01077-x