Abstract
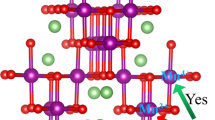
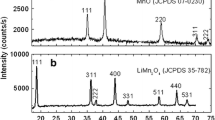
Spinel-type lithium manganese oxides are considered as promising cathode materials for lithium-ion batteries. Trace amounts of Li2MnO3 usually occur as a secondary phase in lithium-manganese spinels in the common high-temperature, solid-state synthesis, affecting the overall Li–Mn stoichiometry in the spinel phase and thereby the electrochemical performance. However, the formation of Li2MnO3 lower than 1 wt.% can hardly be quantified by the conventional analytical techniques. In this work, we synthesized lithium-manganese spinels with different Li/Mn molar ratios and demonstrate that electron paramagnetic resonance (EPR) enables quantifying trace amounts of Li2MnO3 below 10−2 wt.% in the synthesized products. The results reveal that the formation of Li2MnO3 secondary phase is favored by lithium excess in the synthesis. Based on the quantitative evaluation of the EPR data, precise determining Li–Mn stoichiometry in the spinel phase in Li1+xMn2−xO4 materials can be assessed. Accordingly, it is possible to estimate the amount of lithium on 16d-sites in the Li-rich manganese spinels.









Similar content being viewed by others
References
G.E. Blomgren, J. Electrochem. Soc. 164, A5019 (2017)
M.S. Whittingham, Chem. Rev. 104, 4271 (2004)
M.M. Thackeray, W.I.F. David, P.G. Bruce, J.B. Goodenough, Mater. Res. Bull. 18, 461 (1983)
M.M. Doeff, in Encyclopedia of Sustainability Science and Technology, (Springer, New York, 2012), pp. 708–739
B.L. Ellis, K.T. Lee, L.F. Nazar, Chem. Mater. 22, 691 (2010)
M.M. Thackeray, Prog. Solid State Chem. 25, 1 (1997)
R. Gummow, A. De Kock, M. Thackeray, Solid State Ionics 69, 59 (1994)
M. Bianchini, E. Suard, L. Croguennec, C. Masquelier, J. Phys. Chem. C 118, 25947 (2014)
G. Amatucci, J.-M. Tarascon, J. Electrochem. Soc. 149, K31 (2002)
C. Masquelier, M. Tabuchi, K. Ado, R. Kanno, Y. Kobayashi, Y. Maki, O. Nakamura, J.B. Goodenough, J. Solid State Chem. 266, 255 (1996)
D.Y.W. Yu, K. Yanagida, Y. Kato, H. Nakamura, J. Electrochem. Soc. 156, A417 (2009)
V. Massarotti, J. Solid State Chem. 128, 80 (1997)
G. Jain, J. Yang, M. Balasubramanian, J.J. Xu, Chem. Mater. 17, 3850 (2005)
C.S. Johnson, N. Li, J.T. Vaughey, S.A. Hackney, M.M. Thackeray, Electrochem. Commun. 7, 528 (2005)
S. Ivanova, E. Zhecheva, D. Nihtianova, M. Mladenov, R. Stoyanova, J. Alloys Compd. 561, 252 (2013)
S.F. Amalraj, D. Sharon, M. Talianker, C.M. Julien, L. Burlaka, R. Lavi, E. Zhecheva, B. Markovsky, E. Zinigrad, D. Kovacheva, R. Stoyanova, D. Aurbach, Electrochim. Acta 97, 259 (2013)
E. Erdem, V. Mass, A. Gembus, A. Schulz, V. Liebau-Kunzmann, C. Fasel, R. Riedel, R.-A. Eichel, Phys. Chem. Chem. Phys. 11, 5628 (2009)
P. Jakes, E. Erdem, A. Ozarowski, J. van Tol, R. Buckan, D. Mikhailova, H. Ehrenberg, R.-A. Eichel, Phys. Chem. Chem. Phys. 13, 9344 (2011)
R.-A.E.P. Jakes, J. Granwehr, H. Kungl, Z. Phys. Chem. 229, 1439 (2015)
P. Jakes, L. Kröll, A. Ozarowski, J. van Tol, D. Mikhailova, H. Ehrenberg, R.-A. Eichel, Z. Phys. Chem. 231, 905 (2017)
P. Jakes, G. Cohn, Y. Ein-Eli, F. Scheiba, H. Ehrenberg, R.-A. Eichel, ChemSusChem 5, 2278 (2012)
J. Wandt, C. Marino, H.A. Gasteiger, P. Jakes, R.-A. Eichel, J. Granwehr, Energy Environ. Sci. 8, 1358 (2015)
A. Niemöller, P. Jakes, S. Kayser, Y. Lin, W. Lehnert, J. Granwehr, J. Magn. Reson. 269, 157 (2016)
J. Wandt, P. Jakes, J. Granwehr, H.A. Gasteiger, R.A. Eichel, Angew. Chem. Int. Ed. 128, 7006 (2016)
A. Niemöller, P. Jakes, S. Eurich, A. Paulus, H. Kungl, J. Chem. Phys. 148, 14705 (2018)
S. Mandal, R.M. Rojas, J.M. Amarilla, P. Calle, N.V. Kosova, V.F. Anufrienko, J.M. Rojo, Chem. Mater. 14, 1598 (2002)
M. Kopeć, J.R. Dygas, F. Krok, A. Mauger, F. Gendron, B. Jaszczak-Figiel, A. Gagor, K. Zaghib, C.M. Julien, Chem. Mater. 21, 2525 (2009)
R.K. Stoyanova, E.N. Zhecheva, M.Y. Gorova, J. Mater. Chem. 10, 1377 (2000)
E. Zhecheva, R. Stoyanova, Solid State Commun. 135, 405 (2005)
P.W. Anderson, P.R. Weiss, Rev. Mod. Phys. 25, 269 (1953)
M.M. Thackeray, J. Electrochem. Soc. 139, 363 (1992)
D. Capsoni, M. Bini, G. Chiodelli, V. Massarotti, M.C. Mozzati, C.B. Azzoni, Solid State Commun. 125, 179 (2003)
Acknowledgements
We gratefully acknowledge funding from the German Federal Ministry of Education and Research (BMBF-project DESIREE, Grant no. 03SF0477A). S.Y. and U.S. furthermore acknowledge financial support by the research training group “MobilEM” funded by the German Research Foundation. In addition, we thank Prof. Josef Granwehr for the vivid discussion about this research project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, R., Jakes, P., Eurich, S. et al. Secondary-Phase Formation in Spinel-Type LiMn2O4-Cathode Materials for Lithium-Ion Batteries: Quantifying Trace Amounts of Li2MnO3 by Electron Paramagnetic Resonance Spectroscopy. Appl Magn Reson 49, 415–427 (2018). https://doi.org/10.1007/s00723-018-0983-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-018-0983-4