Abstract
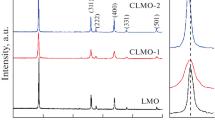
In this study, LMO-xNa and LNMO-yAl were synthesized by a simple high-temperature solid-phase method. MnO2, Li2CO3, NaOH, and Al2O3 were used as manganese source, lithium source, sodium source and aluminum source, respectively. The effects of Na doping and Na, Al co-doping on the electrochemical performance and crystal structure of spinel LiMn2O4 materials were explored, and the crystal parameters and cycle performance of the co-doped materials were discussed emphatically. Through the discussion of SEM, XRD, EIS curve, cycle curve and the performance of the first charge and discharge cycle, it was concluded that when the Na doping ratio x = 0.15 and the Al doping ratio y = 0.10, the reversible cycle capacity of the material maintained is as high as 95%, and the first discharge capacity reaches 125.5 m Ah g–1. It can be seen that the Na, Al co-doped LNMO-yAl not only maintains a certain discharge capacity and has a higher capacity retention rate, it is a promising cathode material for lithium-ion batteries.






Similar content being viewed by others
REFERENCES
G. L. Tian, J. Chem. Ind. 36, 31 (2018). https://doi.org/10.3969/j.issn.1673-9647.2018.06.007
W. Tang, Y. Hou, F. Wang, et al., J. Nano Lett. 13, 2036 (2013). https://doi.org/10.1021/nl400199
S. Huang, Z. Wen, J. Zhang, et al., Solid State Ionics 177, 851 (2006). https://doi.org/10.1016/j.ssi.2006.01.050
R. Dominko, M. Gaberscek, M. Bele, et al., J. Eur. Ceram. Soc. 27, 909 (2007). https://doi.org/10.1016/j.jeurceramsoc.2006.04.133
H. Liu, Y. Feng, K. Wang, et al., J. Phys. Chem. Solids 69, 2037 (2008). https://doi.org/10.1016/j.jpcs.2008.02.017
J. Huang and Z. Jiang, J. Electrochim. Acta 53, 7756 (2008). https://doi.org/10.1016/j.electacta.2008.05.03
L. Yang and L. Gao, J. Alloys Compd. 485, 93 (2009). https://doi.org/10.1016/j.jallcom.2009.05.151
J. Wolfenstine, U. Lee, and J. L. Allen, J. Power Sources 154, 287 (2006). https://doi.org/10.1016/j.jpowsour.2005.12.044
T. Paulos, G. Haftu, V. Veeraiah, et al., J. Korean Phys. Soc. 76, 940 (2020). https://doi.org/10.3938/jkps.76.940
C. Y. Zhu, J. X. Liu, X. H. Yu, Y. J. Zhang, et al., J. Ceram. Int. 06, 187 (2019). https://doi.org/10.1016/j.ceramint.2019.06.187
S. Bhuvaneswari, U. V. Varadaraju, and R. Gopalan, J. Electrochim. Acta 301, 342 (2019). https://doi.org/10.1016/j.electacta.2019.01.174
J. B. Jiang, W. Li, H. J. Deng, et al., J. Nanosci. Nanotechnol. 19, 125 (2019). https://doi.org/10.1166/jnn.2019.16386
Y. H. Hou, K. Chang, H. W. Tang, et al., Electrochim. Acta 319, 587 (2019). https://doi.org/10.1016/j.electacta.2019.07.016
H. Y. Zhao, Y. F. Nie, D. Y. Que, et al., Materials 2807, 17 (2019). https://doi.org/10.3390/ma12172807
G. Li, X. Chen, Y. L. Yu, B. Zhang, and W. S. Yang, J. Solid State Electron. 22, 3099 (2018). http://dx.chinadoi.cn/10.1007/s10008-018-4016-x
W. Zhang, X. L. Sun, Y. X. Tang, et al., J. Am. Chem. Soc. 141, 14038 (2019). https://doi.org/10.1021/jacs.9b05531
M. Michalska, D. A. Złkowska, J. B. Jasinskib, et al., J. Electrochim. Acta 276, 37 (2018). https://doi.org/10.1016/j.electacta.2018.04.165
S. M. Wang, H. L. Liu, M. W. Xiang, et al., J. New J. Chem. 44, 10569 (2020). https://doi.org/10.1039/D0NJ01290D
K. Chudzika, M. Świětosławski, M. Bakierska, et al., J. Appl. Surf. Sci. 147138, 531 (2020). https://doi.org/10.1016/j.apsusc.2020.147138
M. Bakierska, M. Świětosławski, K. Chudzik, et al., Solid State Ionics 317, 190 (2018). https://doi.org/10.1016/j.ssi.2018.01.014
Z. R. Yang, Y. J. Wang, X. C. Chen, et al., Chem. Sel. 4, 9583 (2019). https://doi.org/10.1002/slct.201902685
Y. N. Zhang, Y. J. Zhang, M. Y. Zhang, et al., J. Mater. 71, 608 (2019). https://doi.org/10.1007/s11837-018-2873-5
C. Z. Fei, M. Y. Zhou, and H. X. Ning, J. Energy Storage 101036, 27 (2020). https://doi.org/10.1016/j.est.2019.101036
M. J. Lindsay, J. X. Wang, and H. K. Liu, J. Power Sources 119–121, 84 (2003). https://doi.org/10.1016/S0378-7753(03)00130-7
Y. Hamon, T. Brousse, F. Jousse, et al., J. Power Sources 97, 185 (2001). https://doi.org/10.1016/S0378-7753(01)00616-4
T. Kakuda, K. Uematsu, K. Toda, and M. Sato, J. Power Sources 167, 499 (2007). https://doi.org/10.1016/j.jpowsour.2007.01.035
M. A. Kebede, M. J. Phasha, et al., J. Sustain. Energy Technol. 5, 4 (2014). https://doi.org/10.1016/j.seta.2013.11.005
Y. S. Lee, N. Kumada, and M. Yoshio, J. Power Sources 96, 376 (2001). https://doi.org/10.1016/S0378-7753(00)00652-2
T. Yi, X. Hu, and K. Gao, J. Power Sources 162, 636 (2006). https://doi.org/10.1016/j.jpowsour.2006.07.019
L. Xiao, Y. Zhao, Y. Yang, Y. Cao, et al., Electrochim. Acta 54, 545 (2008). https://doi.org/10.1016/j.electacta.2008.07.037
A. B. Yuan, L. Tian, W. M. Xu, and Y. Q. Wang, J. Power Sources 195, 5032 (2010). https://doi.org/10.1016/j.jpowsour.2010.01.074
H. W. Xiao, Y. R. Wang, K. Xie, et al., J. Alloys Compd. 738, 25 (2018). https://doi.org/10.1016/j.jallcom.2017.12.143
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zhao, Zy., Liu, X. & Shao, Zc. Solid-State Synthesis of Na and Al Co-doped Lithium Manganese Spinel Cathode Material. Russ. J. Phys. Chem. 96 (Suppl 1), S183–S189 (2022). https://doi.org/10.1134/S0036024422140321
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422140321