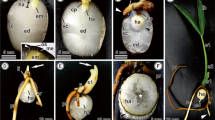
Abstract
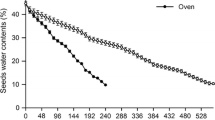
The cotyledonary petiole (CP) completely envelops the embryo axis during embryogenesis in Arecaceae. There is little information available, however, on the roles of that structure in seed germination and initial seedling development—crucial plant life cycle phases. The study therefore sought to evaluate the roles of CP in the germination and post-seminal development of the recalcitrant seeds of Mauritia flexuosa, an ecologically and economically important neotropical palm. The CP and the embryo/vegetative axis were evaluated during germination and initial seedling development using standard morphological, anatomical, histochemical, and ultrastructural methodologies. Evaluations of dormant seeds incubated for 60 days were also performed. The CP (a) promotes seedling protrusion in the germination, extending the embryo axis outside the seed; (b) protects the vegetative axis through the development of coating rich in phenolic compounds and lignin; (c) participates in reserve translocation, with the conversion of its own proteinaceous/mucilaginous reserves into transitional starch, as well as acting in the transport of endospermic reserves; (d) favors aeration, with the formation of pathways among stomata, substomatal chambers, and intercellular spaces; (e) controls seedling morphogenesis by modulating the curvature of the vegetative axis; and (f) contributes to overcoming seed bank dormancy through cytological alterations (protein synthesis and mitochondrial proliferation). The cotyledonary petiole of palms is a unique and multifunctional structure among angiosperms, with crucial roles in germination and seedling establishment.












Similar content being viewed by others
References
Baker WJ, Dransfield J (2016) Beyond genera Palmarum: progress and prospects in palm systematics. Bot J Linn Soc 182:207–233. https://doi.org/10.1111/boj.12401
Baskin CC, Baskin JM (2014) Seeds: ecology, biogeography, and evolution of dormancy and germination. Elsevier/AP, San Diego
Bewley JD, Bradford KJ, Hilhorst HWM, Nonogaki H (2013) Seeds: physiology of development, germination and dormancy, 3rd edn. Springer, New York
Brasil (Ministério da Agricultura Pecuária e Abastecimento) (2009) Regras para análise de sementes. Mapa/ACS, Brasília, DF
Carvalho VS, Ribeiro LM, Lopes PSN, Agostinho CO, Matias LJ, Mercadante-Simões MO, Correia LNF (2015) Dormancy is modulated by seed structures in palms of the cerrado biome. Aust J Bot 63:444. https://doi.org/10.1071/BT14224
DeMason DA (1988) Embryo structure and storage reserve histochemistry in the palm Washingtonia filifera. Am J Bot 75:330. https://doi.org/10.2307/2443980
Demason DA, Thomson WW (1981) Structure and ultrastructure of the cotyledon of date palm (Phoenix dactylifera L.). Bot Gaz 142:320–328. https://doi.org/10.1086/337230
DeMason DA, Sexton R, Gorman M, Reid JSG (1985) Structure and biochemistry of endosperm breakdown in date palm (Phoenix dactylifera L.) seeds. Protoplasma 126:159–167. https://doi.org/10.1007/BF01281791
Dias DS, Ribeiro LM, Lopes PSN, Munné-Bosch S, Garcia QS (2017) Hormonal profile and the role of cell expansion in the germination control of Cerrado biome palm seeds. Plant Physiol Biochem 118:168–177. https://doi.org/10.1016/j.plaphy.2017.06.015
Dias DS, Ribeiro LM, Lopes PSN, Melo GA, Müller M, Munné-Bosch S (2018) Haustorium–endosperm relationships and the integration between developmental pathways during reserve mobilization in Butia capitata (Arecaceae) seeds. Ann Bot 122:267–277. https://doi.org/10.1093/aob/mcy065
Dickison WC (2000) Integrative plant anatomy. Harcourt/Academic Press, San Diego
Dransfield J, Uhl NW, Asmussen CBA, Baker WJ, Harley MM, Lewis CE (2008) Genera Palmarum: the evolution and classification of palms, New ed. Kew Publ. Royal Botanical Garden, Kew
Feder N, O’Brien TP (1968) Plant microtechnique: some principles and new methods. Am J Bot 55:123. https://doi.org/10.2307/2440500
Finch-Savage WE, Leubner-Metzger G (2006) Seed dormancy and the control of germination: Tansley review. New Phytol 171:501–523. https://doi.org/10.1111/j.1469-8137.2006.01787.x
Gatin CL (1906) Recherches anatomiques et chimiques sur la germinacion des palmiers. Ann Sci Nat Bot 3:191–314
Genovese-Marcomini PR, Mendonça SM, Carmello-Guerreiro SM (2013) Embryonic development of Syagrus inajai (Spruce) Becc. (Arecaceae, Arecoideae), an Amazonian palm. Aust J Bot 61:611. https://doi.org/10.1071/BT13162
Haccius B, Philip VJ (1979) Embryo development in Cocos nucifera L.: a critical contribution to a general understanding of palm embryogenesis. Plant Syst Evol 132:91–106
Henderson A (2002) Evolution and ecology of palms. New York Botanical Garden Press, Bronx
Henderson FM (2006) Morphology and anatomy of palm seedlings. Bot Rev 72:273–329. https://doi.org/10.1663/0006-8101(2006)72[273:MAAOPS]2.0.CO;2
Horn CM, Gilmore MP, Endress BA (2012) Ecological and socio-economic factors influencing aguaje (Mauritia flexuosa) resource management in two indigenous communities in the Peruvian Amazon. For Ecol Manag 267:93–103. https://doi.org/10.1016/j.foreco.2011.11.040
Johansen DA (1940) Plant microtechnique. McGraw-Hill, New York
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137A–138A
Kubitzki K (1998) Flowering plants, monocotyledons. Springer, Berlin, Heidelberg
Linkies A, Graeber K, Knight C, Leubner-Metzger G (2010) The evolution of seeds. New Phytol 186:817–831. https://doi.org/10.1111/j.1469-8137.2010.03249.x
Long RL, Gorecki MJ, Renton M, Scott JK, Colville L, Goggin DE, Commander LE, Westcott DA, Cherry H, Finch-Savage WE (2015) The ecophysiology of seed persistence: a mechanistic view of the journey to germination or demise: the ecophysiology of seed persistence. Biol Rev 90:31–59. https://doi.org/10.1111/brv.12095
Lorenzi H, Noblick L, Kahn F, Ferreira E (2010) Flora Brasileira: Arecaceae (palmeiras). Instituto Plantarum, Nova Odessa
Marques A, Buijs G, Ligterink W, Hilhorst H (2018) Evolutionary ecophysiology of seed desiccation sensitivity. Funct Plant Biol 45:1083. https://doi.org/10.1071/FP18022
Mazzottini-dos-Santos HC, Ribeiro LM, Mercadante-Simões MO, Sant’Anna-Santos BF (2015) Ontogenesis of the pseudomonomerous fruits of Acrocomia aculeata (Arecaceae): a new approach to the development of pyrenarium fruits. Trees 29:199–214. https://doi.org/10.1007/s00468-014-1104-0
Mazzottini-dos-Santos HC, Ribeiro LM, Oliveira DMT (2017) Roles of the haustorium and endosperm during the development of seedlings of Acrocomia aculeata (Arecaceae): dynamics of reserve mobilization and accumulation. Protoplasma 254:1563–1578. https://doi.org/10.1007/s00709-016-1048-x
Mazzottini-dos-Santos HC, Ribeiro LM, Oliveira DMT (2018) Structural changes in the micropylar region and overcoming dormancy in Cerrado palms seeds. Trees 32:1415–1428. https://doi.org/10.1007/s00468-018-1723-y
Melo WA, Freitas CG, Bacon CD, Collevatti RG (2018) The road to evolutionary success: insights from the demographic history of an Amazonian palm. Heredity 121:183–195. https://doi.org/10.1038/s41437-018-0074-1
Neves S d C, Ribeiro LM, da Cunha IRG, Pimenta MAS, Mercadante-Simões MO, Lopes PSN (2013) Diaspore structure and germination ecophysiology of the babassu palm (Attalea vitrivir). Flora 208:68–78. https://doi.org/10.1016/j.flora.2012.12.007
O’Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373
Oliveira NCC, Lopes PSN, Ribeiro LM, Mercandante-Simões MO, Oliveira LAA, Silvério FO (2013) Seed structure, germination, and reserve mobilization in Butia capitata (Arecaceae). Trees 27:1633–1645. https://doi.org/10.1007/s00468-013-0910-0
Orozco-Segovia A, Batis AI, Roja-Aréchiga M, Mendoza A (2003) Seed biology of palms: a review. Palms 47:79–94
Paiva EAS, Pinho SZ, Oliveira DMT (2011) Large plant samples: how to process for GMA embedding? In: Chiarini-Garcia H, Melo RCN (eds) Light microscopy: methods and protocols. Humana Press, Totowa, pp 37–49
Panza V, LáInez V, Maldonado S (2004) Seed structure and histochemistry in the palm Euterpe edulis. Bot J Linn Soc 145:445–453. https://doi.org/10.1111/j.1095-8339.2004.00293.x
Pizzolato P, Lillie RD (1973) Mayer’s tannic acid-ferric chloride stain for mucins. J Histochem Cytochem 21:56–64
Porto KCN, Nunes YRF, Ribeiro LM (2018) The dynamics of recalcitrant seed banks of Mauritia flexuosa (Arecaceae) reveal adaptations to marsh microenvironments. Plant Ecol 219:199–207. https://doi.org/10.1007/s11258-017-0788-9
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Ribeiro LM, Souza PP, Rodrigues AG Jr, Oliveira TGS, Garcia QS (2011) Overcoming dormancy in macaw palm diaspores, a tropical species with potential for use as bio-fuel. Seed Sci Technol 39:303–317. https://doi.org/10.15258/sst.2011.39.2.04
Ribeiro LM, Oliveira DMT, Garcia Q de S (2012) Structural evaluations of zygotic embryos and seedlings of the macaw palm (Acrocomia aculeata, Arecaceae) during in vitro germination. Trees 26:851–863. https://doi.org/10.1007/s00468-011-0659-2
Ribeiro LM, Garcia QS, Müller M, Munné-Bosch S (2015) Tissue-specific hormonal profiling during dormancy release in macaw palm seeds. Physiol Plant 153:627–642. https://doi.org/10.1111/ppl.12269
Robards AW (1978) An introduction to techniques for scanning electron microscopy of plant cells. In: Hall JL (ed) Electron microscopy and cytochemistry of plant cells. Elsevier, New York, pp 343–403
Roland AM (1978) General preparations and staining of thin sections. In: Hall JL (ed) Electron microscopy and cytochemistry of plant cells. Elsevier, New York, pp 1–62
Silva RS, Ribeiro LM, Mercadante-Simões MO, Nunes YRF, Lopes PSN (2014) Seed structure and germination in buriti (Mauritia flexuosa), the swamp palm. Flora 209:674–685. https://doi.org/10.1016/j.flora.2014.08.012
Souza JN, Ribeiro LM, Mercadante-Simões MO (2017) Ontogenesis and functions of saxophone stem in Acrocomia aculeata (Arecaceae). Ann Bot 119:353–365. https://doi.org/10.1093/aob/mcw215
Steinbrecher T, Leubner-Metzger G (2017) The biomechanics of seed germination. J Exp Bot 68:765–783. https://doi.org/10.1093/jxb/erw428
Steinbrecher T, Leubner-Metzger G (2018) Tissue and cellular mechanics of seeds. Curr Opin Genet Dev 51:1–10. https://doi.org/10.1016/j.gde.2018.03.001
Tillich H-J (2007) Seedling diversity and the homologies of seedling organs in the order Poales (monocotyledons). Ann Bot 100:1413–1429. https://doi.org/10.1093/aob/mcm238
Tomlinson PB (1990) The structural biology of palms. Oxford: Clarendon Press; Oxford University Press
Tomlinson PB (2006) The uniqueness of palms. Bot J Linn Soc 151:5–14. https://doi.org/10.1111/j.1095-8339.2006.00520.x
Veloso VHS, Ribeiro LM, Mercadante-Simões MO, Nunes YRF (2016) Cytological aspects of recalcitrance in dormant seeds of Mauritia flexuosa (Arecaceae). Acta Physiol Plant 38:171. https://doi.org/10.1007/s11738-016-2194-7
Vidal BC (1970) Dichroism in collagen bundles stained with xylidine-Ponceau 2R. Ann Histochim 15:289–296
Watson ML (1958) Staining of tissue sections for electron microscopy with heavy metals II. Application of solutions containing lead and barium. J Biophys Biochem Cytol 4:727–730
Werker E (1997) Seed anatomy. Gebrüder Borntraeger, Berlin
Acknowledgements
The authors thank the Centro de Microscopia da Universidade Federal de Minas Gerais—CM/UFMG for the electron microscopy analyses.
Funding
The authors received from the Fundação de Amparo à Pesquisa do Estado de Minas Gerais—Fapemig financial support for the research project (Process: APQ-00468-1) and from Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq the Master’s degree scholarship awarded to ACFM, under the Programa de Pesquisa Ecológica de Longa Duração—PELD-VERE (Process: 441440/2016-9), and research productivity grants awarded to LMR (Process: 304627/2015-1), MOMS (Process: 304801/2016-0), and YRFN (Process: 306375/2016-8).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Peter Nick
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Longitudinal sections of the medullary region of the radicles and roots of Mauritia flexuosa submitted to histochemical tests. The drawings on the left represent stages of embryonic development after the cultivation of a seed without an operculum; red indicates the cotyledonary petiole and the germinative button. (a, e, i, m, q) Proteins, revealed by red staining with Xylidine-Ponceau (XP). (b, f, j, n, r) Mucilage, revealed by black staining with tannic acid. (c, g, k, o, s) Starch, revealed by black staining with Lugol’s solution. (d, h, l, p, t) Neutral polysaccharides, revealed by magenta staining with PAS. (a, b) Cells with large protein and mucilaginous reserves, respectively. (c) Absence of starch. (d) Neutral polysaccharide reserves in the cell walls and vacuoles (black arrows). (e, f) Vacuolation (white arrows), indicating early mobilization of proteins and mucilage. (g, h) Accumulations of starch grains. (i–l) Protein residues, indicating the intensification of mobilization associated with starch accumulation and vacuolation (white arrow). (m) Protein residues and vacuolation (white arrow). (n–p) Accumulations of mucilage associated with partial starch mobilization. (q–t) Total protein mobilization associated with vacuolation (white arrow) and starch accumulation. mu, mucilage; nu, nuclei; pi, phenolic idioblast; pt, protein; st, starch; ve, vessel element. (PNG 7036 kb)
ESM 2
Longitudinal sections of the cotyledonary petioles of embryos and seedlings of Mauritia flexuosa submitted to histochemical tests. The drawings on the left represent the stages of embryonic development after cultivation of a seed without an operculum; red indicates the cotyledonary petiole and the germinative button. (a, e, i, m, q) Proteins, stained red with Xylidine-Ponceau (XP). (b, f, j, n, r) Mucilage, stained black by tannic acid. (c, g, k, o, s) Starch, stained black with Lugol’s solution. (d, h, l, p, t) Neutral polysaccharides, stained magenta with PAS. (a, b) Cells with large protein and mucilaginous reserves, respectively. (c) Absence of starch. (d) Reserves of neutral polysaccharides in the cell walls and vacuoles (black arrows). (e, f) Vacuolation (white arrows), indicating early mobilization of proteins and mucilage. (g, h) Accumulations of small starch grains. (i–l) Voluminous vacuoles (white arrows) and protein residues, indicating the intensification of mobilization, associated with starch accumulations. (m) Protein residues and vacuolation (white arrows). (n–p) Accumulations of mucilage, associated with the beginning of starch mobilization. (q–t) Total protein mobilization, vacuolation (white arrows), and partial mobilization of starch; residues of polysaccharides can be observed in the vacuoles (black arrows). mu, mucilage; nu, nuclei; pi, phenolic idioblast; pt, protein; rp, raphide; st, starch. (PNG 6908 kb)
ESM 3
Longitudinal sections of the medullary regions of Mauritia flexuosa radicles obtained from dormant seeds 60 days after sowing and submitted to histochemical tests. The drawing to the left represents the morphology of the embryo; the opercular integument is indicated in brown, and the cotyledonary petiole in red. (a) Proteins, stained red with Xylidine-Ponceau (XP). (b) Mucilage, stained black with tannic acid. (c) Starch, stained black with Lugol’s solution. (d) Neutral polysaccharides, stained magenta with PAS. (a, b) Cells containing abundant protein and mucilage reserves, respectively. (c) Absence of starch. (d) Neutral polysaccharides stored in the cell wall. mu, mucilage; nu, nuclei; pt, protein. (PNG 1393 kb)
ESM 4
Longitudinal sections of the cotyledonary petiole of Mauritia flexuosa obtained from dormant seeds 60 days after sowing and submitted to histochemical tests. The drawing to the left represents the morphology of the embryo; the opercular seed coat is highlighted in brown, and the cotyledonary petiole in red. (a) Proteins, stained red with Xylidine-Ponceau (XP). (b) Mucilage, stained black with tannic acid. (c) Starch, stained black with Lugol’s solution. (d) Neutral polysaccharides, stained magenta with PAS. Cells containing abundant protein and mucilage reserves respectively. (c) Absence of starch. (d) Neutral polysaccharides stored in cell walls and vacuoles (black arrows). mu, mucilage; pt, protein. (PNG 1390 kb)
Rights and permissions
About this article
Cite this article
Ferreira Moura, A.C., Ribeiro, L.M., Mazzottini-dos-Santos, H.C. et al. Cytological and histochemical evaluations reveal roles of the cotyledonary petiole in the germination and seedling development of Mauritia flexuosa (Arecaceae). Protoplasma 256, 1299–1316 (2019). https://doi.org/10.1007/s00709-019-01375-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-019-01375-1