Abstract
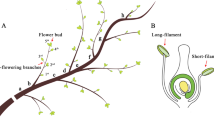
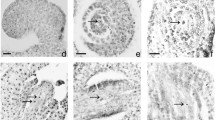
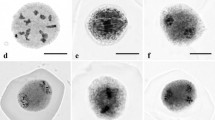
Zingiber officinale Roscoe, the common ginger, is an invaluable horticultural crop cultivated majorly in China, India, Brazil, Jamaica and Nigeria. Its pungent aromatic rhizome is used all over the world as a spice, culinary herb, condiment, home remedy and medicinal agent. This species does not produce seeds and so is difficult to breed its new genotypes through sexual hybridization. Despite the economic and genetic value, ginger has not been subjected to detailed cytogenetic research, which could lead to a better understanding of its reproduction for future genetic improvement. Therefore, the present work is undertaken for the first time to study microsporogenesis and pollen formation in Z. officinale. Pre-meiotic stages were observed under light microscope using anther squashes and staining by either acetocarmine or carbol fuchsin, whereas, the post meiotic stages were visualized under fluorescence microscope using either isolated microspores or pollen grains stained by 4′, 6′-diamidino-2-phenylindole. The combination of these two different cytological techniques allowed the study of the complete microsporogenesis and pollen formation processes step by step starting from pollen mother cells (PMCs) stage to mature pollen grain. Assessment of meiotic behavior was also performed by evaluating all possible PMCs on each slide and at all stages of meiosis. Cytological analysis revealed that only anthers from flowers of small spikes presented appropriate PMCs to analyze meiotic irregularities. The present paper enriches the database of cytology and pollen viability estimations for supporting sexual hybridization. The present study demonstrated a good relationship between the development stage of microspore and the size of the spike. This basic information will hopefully allow the development of protocol for production of androgenic haploids to accelerate breeding and genetic improvement of ginger. Further, research is under progress for evaluation of several media and other cultural factors to achieve microspore embryogenesis and plant regeneration in this species.



























Similar content being viewed by others
References
Adaniya S (2001) Optimal pollination environment of tetraploid ginger (Zingiber officinale Roscoe) evaluated by in vitro pollen germination and pollen tube growth in styles. Sci Hort 90:219–226
Adaniya S, Shirai D (2001) In vitro induction of tetraploid ginger (Zingiber officinale Roscoe) and its pollen fertility and geminability. Sci Hort 88:277–287
Adaniya S, Shoda M (1998a) Variation in pollen fertility and germinability in ginger (Zingiber officinale Roscoe). J Japan Soc Hort Sci 67:872–874
Adaniya S, Shoda M (1998b) Meiotic irregularity in ginger (Zingiber officinale Roscoe). Chromosome Sci 2:141–144
Aybeke M (2012) Anther wall and pollen development in Ophyrys mammosa L. Plant Syst Evol 298:1015–1023
Bhojwani SS, Bhatnagar SP (1999) The embryology of angiosperms. Vikas Publishing House Pvt Ltd, New Delhi
Coleman AW, Goff LJ (1985) Applications of fluorochromes to pollen biology. 1 Mithramycin and 4′ 6′ diamidino-2-phenylindole (DAPI) as vital stains and for quantitative of nuclear DNA. Stain Technol 60:145–154
Dunwell JM (2010) Haploids in flowering plants: origins and exploitation. Plant Biotech J 8:377–424
Fan Z, Aarmstrong KK, Keller WA (1988) Development of microspores in vivo and in vitro in Brassica napus L. Protoplasma 147:191–199
Ferrie AMR, Caswell KL (2011) Isolated microspore culture techniques and recent progress for haploid and doubled haploid plant production. Plant Cell, Tissue Organ Cult 104:301–309
Germana MA (2011a) Anther culture for haploid and doubled haploid production. Plant Cell Tissue Org Cult 104:283–300
Germana MA (2011b) Gametic embryogenesis and haploid technology as a valuable support to plant breeding. Plant Cell Rep 30:839–857
Heslop-Harrison J, Heslop-Harrison Y (1970) Evaluation of pollen viability by enzymatically induced fluorescence: intracellular hydrolysis of fluorescein diacetate. Stain Technol 45:115–120
Heslop-Harrison J, Heslop-Harrison Y, Shivanna KR (1984) The evaluation of pollen quality and a further appraisal of the fluorochromatic (FCR) test procedure. Theor Appl Genet 67:367–375
Jayachandran B, Vijaygopal K, Sethumadhavan P (1979) Floral biology of ginger, Zingiber officinale R. Agri Res J Kerala 17:93–94
Johansen DA (1940) Plant microtechnique. Mc Graw-Hill, London
Kernan Z, Ferrie AMR (2006) Microspore embryogenesis and the development of a double haploidy protocol for cow cockle (Saponaria vaccaria). Plant Cell Rep 25:274–280
Mangaly JK, Nayar J (1990) Palynology of South Indian Zingiberaceae. Bot J Linn Soc 103:351–366
Maraschin SF, dePriester W, Spaink HP, Wang M (2005) Androgenic switch: an example of plant embryogenesis from the male gametophytic perspective. J Exp Bot 56:1711–1726
Mascarenhas JP (1975) The biochemistry of angiosperms pollen development. Bot Rev 41:259–314
Nirmal Babu K, Samsudeen K, Ratnambal MJ (1992) In vitro plant regeneration from leaf-derived callus in ginger (Zingiber officinale Roscoe). Plant Cell Tissue Org Cult 29:71–74
Pagliarini MS (2000) Meiotic behaviour of economically important plant species: the relationship between fertility and male sterility. Genet Mol Biol 23:997–1002
Pillai PK, Vijayakumar T, Nambiar MC (1978) Flowering behaviour and pollen germination in ginger (Zingiber officinale Rosc.). J Plant Crops 6:12–13
Ramachandran K (1969) Chromosome numbers in Zingiberaceae. Cytologia 34:213–221
Ramachandran K (1982) Polyploidy induced in ginger by colchicine treatment. Curr Sci 51:288–289
Theilade I, Theilade J (1996) Ontogeny of pollen grains in Zingiber spectable (Zingiberaceae). Grana 35:162–170
Theilade I, Maersk-Moller ML, Theilade J, Larsen K (1993) Pollen morphology and structure of Zingiber (Zingiberaceae). Grana 32:338–342
Touraev A, Heberle-Bros E (2003) Anther and microspore culture in tobacco. In: Maluszynski M, Kasha KJ, Froster BP, Szarejkop I (eds) Doubled haploid production in crop plants. Kluwer Academic Publishers, Dordrecht, pp 223–228
Wang YQ, Zhang DX, Chen ZY (2004) Pollen histochemistry and pollen: ovule ratio in Zingiberaceae. Ann Bot 94:583–591
Acknowledgments
The authors are thankful to Dr. Rajkumar Kishore, Manipur University, Imphal, Manipur, India for assisting us in the collection of plant material. Thanks also due to Dr. P. Padma, the Head, Department of English, Yogi Vemana University, Kadapa for critically reading manuscript and English assistance. This work was supported by the Department of Biotechnology (DBT)’s Twining Programme for the North-East (BT/34/NE/TBP/2010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Subbarayudu, S., Shankar Naik, B., Sunitibala Devi, H. et al. Microsporogenesis and pollen formation in Zingiber officinale Roscoe. Plant Syst Evol 300, 619–632 (2014). https://doi.org/10.1007/s00606-013-0907-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-013-0907-6