Abstract
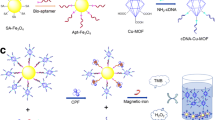
A fluorescence enhancement method is presented for the determination of ochratoxin A (OTA). The interaction of OTA with its aptamer causes structural changes which, in turn, change fluorescence of enzymatically generated polythymine-coated copper nanoparticles (CuNPs) (with excitation/emission maxima at 340/625 nm). The OTA-binding aptamer was immobilized on magnetic beads. When it binds OTA, it is partially released and exposes a region with a partly complimentary DNA strand (cDNA). After magnetic separation, the cDNA was employed as a primer to trigger the terminal deoxynucleotidyl transferase-mediated polymerization. This process generates polythymine which act as a template for synthesis of the CuNPs. The method is sensitive in having a 2.0 nM detection limit for OTA. It was successfully applied to the determination of OTA in spiked diluted red wine.

Schematic presentation of a fluorometric enhancement method for ochratoxin A assay based on ochratoxin A inducing structure switching of its aptamer and enzymatically generated polythymine for copper nanoparticles formation.





Similar content being viewed by others
References
Bayman P, Baker JL, Doster MA, Michailides TJ, Mahoney NE (2002) Ochratoxin production by the Aspergillus ochraceus group and Aspergillus alliaceus. Appl Environ Microbiol 68(5):2326–2329
Duarte SC, Pena A, Lino CM (2010) A review on ochratoxin A occurrence and effects of processing of cereal and cereal derived food products. Food Microbiol 27(2):187–198
Petzinger E, Ziegler K (2000) Ochratoxin A from a toxicological perspective. J Vet Pharmacol Ther 23(2):91–98
Bauer J, Gareis M (1987) Ochratoxin-a in the food-chain. J Vet Med 34(8):613–627
Pfohl-Leszkowicz A, Manderville RA (2007) Ochratoxin A: an overview on toxicity and carcinogenicity in animals and humans. Mol Nutr Food Res 51(1):61–99
Liu JW, Cao ZH, Lu Y (2009) Functional nucleic acid sensors. Chem Rev 109(5):1948–1998
Cruz-Aguado JA, Penner G (2008) Determination of Ochratoxin A with a DNA aptamer. J Agric Food Chem 56(22):10456–10461
Wang Y, Ning G, Wu Y, Wu S, Zeng B, Liu G, He X, Wang K (2018) Facile combination of beta-cyclodextrin host-guest recognition with exonuclease-assistant signal amplification for sensitive electrochemical assay of ochratoxin A. Biosens Bioelectron 124-125:82–88
Zhou L, Sun N, Xu L, Chen X, Cheng H, Wang J, Pei R (2016) Dual signal amplification by an “on-command” pure DNA hydrogel encapsulating HRP for colorimetric detection of ochratoxin A. RSC Adv 6(115):114500–114504
Lin C, Zheng H, Sun M, Guo Y, Luo F, Guo L, Qiu B, Lin Z, Chen G (2018) Highly sensitive colorimetric aptasensor for ochratoxin A detection based on enzyme-encapsulated liposome. Anal Chim Acta 1002:90–96
Lee J, Jeon CH, Ahn SJ, Ha TH (2014) Highly stable colorimetric aptamer sensors for detection of ochratoxin A through optimizing the sequence with the covalent conjugation of hemin. Analyst 139(7):1622–1627
Zhou Z, Hao N, Zhang Y, Hua R, Qian J, Liu Q, Li H, Zhu W, Wang K (2017) A novel universal colorimetric sensor for simultaneous dual target detection through DNA-directed self-assembly of graphene oxide and magnetic separation. Chem Commun (Camb) 53(52):7096–7099
Gillibert R, Triba MN, Lamy de la Chapelle M (2017) Surface enhanced Raman scattering sensor for highly sensitive and selective detection of ochratoxin A. Analyst 143(1):339–345
Zhang G, Zhu C, Huang Y, Yan J, Chen A (2018) A lateral flow strip based Aptasensor for detection of Ochratoxin A in corn samples. Molecules 23(2):291
Lu Z, Chen X, Hu W (2017) A fluorescence aptasensor based on semiconductor quantum dots and MoS2 nanosheets for ochratoxin A detection. Sensors Actuators B Chem 246:61–67
Lv L, Li D, Cui C, Zhao Y, Guo Z (2017) Nuclease-aided target recycling signal amplification strategy for ochratoxin A monitoring. Biosens Bioelectron 87:136–141
Lv L, Li D, Liu R, Cui C, Guo Z (2017) Label-free aptasensor for ochratoxin A detection using SYBR gold as a probe. Sensors Actuators B Chem 246:647–652
Song C, Hong W, Zhang X, Lu Y (2018) Label-free and sensitive detection of Ochratoxin A based on dsDNA-templated copper nanoparticles and exonuclease-catalyzed target recycling amplification. Analyst 143:1829–1834
Dai S, Wu S, Duan N, Wang Z (2016) A luminescence resonance energy transfer based aptasensor for the mycotoxin Ochratoxin A using upconversion nanoparticles and gold nanorods. Microchim Acta 183(6):1909–1916
Liu Y, Yan H, Shangguan J, Yang X, Wang M, Liu W (2018) A fluorometric aptamer-based assay for ochratoxin A using magnetic separation and a cationic conjugated fluorescent polymer. Microchim Acta 185(9):254
Rotaru A, Dutta S, Jentzsch E, Gothelf K, Mokhir A (2010) Selective dsDNA-templated formation of copper nanoparticles in solution. Angew Chem Int 49(33):5665–5667
Song C, Yang X, Wang K, Wang Q, Huang J, Liu J, Liu W, Liu P (2014) Label-free and non-enzymatic detection of DNA based on hybridization chain reaction amplification and dsDNA-templated copper nanoparticles. Anal Chim Acta 827:74–79
Zhang LL, Zhao JJ, Duan M, Zhang H, Jiang JH, Yu RQ (2013) Inhibition of dsDNA-templated copper nanoparticles by pyrophosphate as a label-free fluorescent strategy for alkaline phosphatase assay. Anal Chem 85(8):3797–3801
Chen JH, Liu J, Fang ZY, Zeng LW (2012) Random dsDNA-templated formation of copper nanoparticles as novel fluorescence probes for label-free lead ions detection. Chem Commun 48:1057–1059
Liu H, Ma C, Wang J, Wang K, Wu K (2017) A turn-on fluorescent method for determination of the activity of alkaline phosphatase based on dsDNA-templated copper nanoparticles and exonuclease based amplification. Microchim Acta 184(7):2483–2488
Qing ZH, He XX, He DG, Wang KM, Xu FZ, Qing TP, Yang X (2013) Poly(thymine)-templated selective formation of fluorescent copper nanoparticles. Angew Chem Int 52(37):9719–9722
Ye T, Li C, Su C, Ji X, Zheng J, Tinnefeld P, He Z (2015) Enzymatic polymerization of poly(thymine) for the synthesis of copper nanoparticles with tunable size and their application in enzyme sensing. Chem Commun (Camb) 51(41):8644–8647
Luo L, Xu F, Shi H, He X, Qing T, Lei Y, Tang J, He D, Wang K (2017) Label-free and sensitive assay for deoxyribonuclease I activity based on enzymatically-polymerized superlong poly(thymine)-hosted fluorescent copper nanoparticles. Talanta 169:57–63
Chen J, Xu Y, Ji X, He Z (2017) Enzymatic polymerization-based formation of fluorescent copper nanoparticles for the nuclease assay. Sensors Actuators B Chem 239:262–269
Cao J, Wang W, Bo B, Mao X, Wang K, Zhu X (2017) A dual-signal strategy for the solid detection of both small molecules and proteins based on magnetic separation and highly fluorescent copper nanoclusters. Biosens Bioelectron 90:534–541
Hu W, Ning Y, Kong J, Zhang X (2015) Formation of copper nanoparticles on poly(thymine) through surface-initiated enzymatic polymerization and its application for DNA detection. Analyst 140(16):5678–5684
Covarelli L, Beccari G, Marini A, Tosi L (2012) A review on the occurrence and control of ochratoxigenic fungal species and ochratoxin A in dehydrated grapes, non-fortified dessert wines and dried vine fruit in the Mediterranean area. Food Control 26(2):347–356
Acknowledgements
The authors thank the Natural Science Foundation Project of CQ (No. cstc2018jscx-msybX0263), National Natural Science Foundation of China (No. 21405125), China Agriculture Research System (No. CARS-27) and National Risk Assessment Program for Agricultural Products Quality and Safety (No. GJFP2018003 and GJFP2017013) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1.04 mb)
Rights and permissions
About this article
Cite this article
He, Y., Tian, F., Zhou, J. et al. A fluorescent aptasensor for ochratoxin A detection based on enzymatically generated copper nanoparticles with a polythymine scaffold. Microchim Acta 186, 199 (2019). https://doi.org/10.1007/s00604-019-3314-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3314-z