Abstract
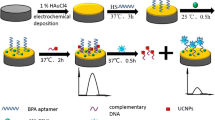
The authors describe a turn-on luminescence resonance energy transfer (LRET) method for the detection of the mycotoxin Ochratoxin A (OTA). It utilizes upconversion nanoparticles (UCNPs) of the type NaYF4: Yb, Er as the energy donor and gold nanorods (Au NRs) as the acceptor. Biotin-labeled OTA aptamers were bound to the surface of the avidin-functionalized UCNPs. The AuNRs, in turn, were modified with thiolated OTA aptamer cDNA via thiol chemistry. The emission band of the UCNPs under 980-nm laser excitation has a maximum peaking at 657 nm and overlaps the absorption band of the AuNRs which peaks at 660 nm. Quenching of luminescence occurs because the hybridization actions shorten the distance between UCNPs and AuNRs. If, however, OTA is added, the two kinds of particles separate again because of the high affinity between OTA and the OTA aptamer. As a result, luminescence is recovered. The calibration plot is linear in the 0.05 to 100 ng mL−1 OTA concentration range, and the limit of detection is 27 pg mL−1. The method was successfully applied to the determination of OTA in beer.

The luminescence of aptamer-modified upconversion nanoparticles (avidin-UCNPs) is quenched by cDNA-modified gold nanorods (AuNRs) due to hybridization. In the presence of Ochratoxin A (OTA), however, the luminescence of the UCNPs recovers due to competitive binding of aptamer to OTA.





Similar content being viewed by others
References
Davis N, Searcy J, Diener U (1969) Production of Ochratoxin A by Aspergillus ochraceus in a Semisynthetic Medium. Appl Microbiol 17(5):742–727
Bellver J, Fernández M, Ruiz M, Juan A (2014) Presence of Ochratoxin A (OTA) Mycotoxin in Alcoholic Drinks from Southern European Countries: Wine and Beer. J Agric Food Chem 62(31):7643–7651
Lai X, Liu R, Ruan C, Zhang H, Liu C (2015) Occurrence of aflatoxins and ochratoxin A in rice samples from six provinces in China. Food Control 50:401–404
Bui-Klimke TR, Wu F (2015) Ochratoxin A and Human Health Risk: A Review of the Evidence. Crit Rev Food Sci 55(13):1860–1869
Majdinasab M, Sheikh-Zeinoddin M, Soleimanian-Zad S, Li P, Zhang Q, Li X, Tang X, Li J (2015) A reliable and sensitive time-resolved fluorescent immunochromatographic assay (TRFICA) for ochratoxin A in agro-products. Food Control 47:126–134
Wang C, Qian J, Wang K, Wang K, Liu Q, Dong X, Wang C, Huang X (2015) Magnetic-fluorescent-targeting multifunctional aptasensorfor highly sensitive and one-step rapid detection of ochratoxin A. Biosens Bioelectron 68:783–790
Lee T, Saad B, Salleh B, Mat I (2013) Micro-solid phase extraction of ochratoxin A, and its determination in urine using capillary electrophoresis. Microchim Acta 180(11–12):1149–1156
Pittet A, Royer D (2002) Rapid, Low Cost Thin-Layer Chromatographic Screening Method for the Detection of Ochratoxin A in Green Coffee at a Control Level of 10 μg/kg. J Agric Food Chem 50:243–247
Flajs D, Domijan A, Ivic D, Cvjetkovic B, Peraica M (2009) ELISA and HPLC analysis of ochratoxin A in red wines of Croatia. Food Control 20(6):590–592
Olsson J, Borjesson T, Lundstedt T, Schnurer J (2002) Detection and quantification of ochratoxin A and deoxynivalenol in barley grains by GC-MS and electronic nose. Int J Food Microbiol 72(3):203–214
Kim S, Lim H (2015) Chemiluminescence immunoassay using magnetic nanoparticles with targeted inhibition for the determination of ochratoxin A. Talanta 140:183–188
Dhesingh R, K V, Norio M (2007) Recent advancements in surface plasmon resonance immunosensors for detection of small molecules of biomedical, food and environmental interest. Sensors Actuators B Chem 121(1):158–177
Liu B, Tsao Z, Wang J, Yu F (2008) Development of a Monoclonal Antibody against Ochratoxin A and Its Application in Enzyme-Linked Immunosorbent Assay and Gold Nanoparticle Immunochromatographic Strip. Anal Chem 80:7029–7035
Chen X, Huang Y, Duan N, Wu S, Xia Y, Ma X, Zhu C, Jiang Y, Ding Z, Wang Z (2014) Selection and characterization of single stranded DNA aptamers recognizing fumonisin B1. Microchim Acta 181(11–12):1317–1324
Yuan F, Chen HQ, Xu J, Zhang YY, Wu Y, Wang L (2014) Aptamer-Based Luminescence Energy Transfer from Near-Infrared-to-Near-Infrared Upconverting Nanoparticles to Gold Nanorods and Its Application for the Detection of Thrombin. Chem Eur J 20(10):2888–2894
Lv Z, Chen A, Liu J, Guan Z, Zhou Y, Xu S, Yang S, Li C (2014) A Simple and Sensitive Approach for Ochratoxin A Detection Using a Label-Free Fluorescent Aptasensor. PLoS One 9(1):e85968
Yang L, Zhang Y, Li R, Lin C, Guo L, Qiu B, Lin Z, Chen G (2015) Electrochemiluminescence biosensor for ultrasensitive determination of ochratoxin A in corn samples based on aptamer and hyperbranched rolling circle amplification. Biosens Bioelectron 70:268–274
Zhu Z, Peng M, Zuo L, Zhu Z, Wang F, Chen L, Li J, Shan G, Luo S (2015) An aptamer based surface plasmon resonance biosensor for the detection of ochratoxin A in wine and peanut oil. Biosens Bioelectron 65:320–326
Mishra R, Hayat A, Catanante G, Istamboulie G, Marty J (2016) Sensitive quantitation of Ochratoxin A in cocoa beans using differential pulse voltammetry based aptasensor. Food Chem 192:799–804
Stanisavljevic M, Krizkova S, Vaculovicova M, Kizek R, Adam V (2015) Quantum dots-fluorescence resonance energy transfer-based nanosensors and their application. Biosens Bioelectron 74:562–574
Li X, Zhang F, Zhao D (2015) Lab on upconversion nanoparticles: optical properties and applications engineering via designed nanostructure. Chem Soc Rev 44(6):1346–1378
Tu D, Zheng W, Liu Y, Zhu H, Chen X (2014) Luminescent biodetection based on lanthanide-doped inorganic nanoprobes. Coord Chem Rev 273:13–29
Zhou F, Noor M, Krull U (2014) Luminescence Resonance Energy Transfer-Based Nucleic Acid Hybridization Assay on Cellulose Paper with Upconverting Phosphor as Donors. Anal Chem 86(5):2719–2726
Saleh S, Ali R, Hirsch T, Wolfbeis O (2011) Detection of biotin-avidin affinity binding by exploiting a self-referenced system composed of upconverting luminescent nanoparticles and gold nanoparticles. J Nanoparticle Res 13(10):4603–4611
Ong L, Ang L, Alonso S, Zhang Y (2014) Bacterial imaging with photostable upconversion fluorescent nanoparticles. Biomater 35(9):2987–2998
Li Z, Lv S, Wang Y, Chen S, Liu Z (2015) Construction of LRET-Based Nanoprobe Using Upconversion Nanoparticles with Confined Emitters and Bared Surface as Luminophore. J Am Chem Soc 137(9):3421–3427
Yang YM (2014) (2014) Upconversion nanophosphors for use in bioimaging, therapy, drug delivery and bioassays. Microchim Acta 181:263–294
Song C, Ye Z, Wang G, Yuan J, Guan Y (2010) Core-Shell Nanoarchitectures: A Strategy To Improve the Efficiency of Luminescence Resonance Energy Transfer. ACS Nano 4(9):5389–5397
Kelkar S, Xue L, Turner S, Reineke T (2014) Lanthanide-containing polycations for monitoring polyplex dynamics via lanthanide resonance energy transfer. Biomacromolecules 15(5):1612–1624
Yang D, Ma P, Hou Z, Cheng Z, Li C, Lin J (2015) Current advances in lanthanide ion (Ln3+)-based upconversion nanomaterials for drug delivery. Chem Soc Rev 44:1416–1448
Mayer K, Hafner J (2011) Localized surface plasmon resonance sensors. Chem Rev 111(6):3828–3857
Li Z, Zhang Y (2008) An efficient and user-friendly method for the synthesis of hexagonal-phase NaYF(4): Yb, Er/Tm nanocrystals with controllable shape and upconversion fluorescence. Nanotechnology 19(34):345606
Wu SJ, Duan N, Shi Z, Fang CC, Wang ZP (2014) Simultaneous Aptasensor for Multiplex Pathogenic Bacteria Detection Based on Multicolor Upconversion Nanoparticles Labels. Anal Chem 86(6):3100–3107
Guo H, Ruan F, Lu L, Hu J, Pan J, Yang Z, Ren B (2009) Correlating the Shape, Surface Plasmon Resonance, and Surface-Enhanced Raman Scattering of Gold Nanorods. J Phys Chem C 113(24):10459–10464
Acknowledgments
This work was partly supported by the National S&T Support Program of China (2015BAD17B02), National Natural Science Foundation of China (21375049, 31401576), JUSRP51309A and Synergetic Innovation Center of Food Safety and Quality Control of Jiangsu Province.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests
Electronic supplementary material
ESM 1
(DOCX 107766 kb)
Rights and permissions
About this article
Cite this article
Dai, S., Wu, S., Duan, N. et al. A luminescence resonance energy transfer based aptasensor for the mycotoxin Ochratoxin A using upconversion nanoparticles and gold nanorods. Microchim Acta 183, 1909–1916 (2016). https://doi.org/10.1007/s00604-016-1820-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1820-9