Abstract
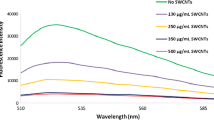
The authors describe an aptamer based assay for the mycotoxin patulin (PAT). A gold electrode was modified with a composite made from ZnO nanorods (ZnO-NRs) and chitosan. The ZnO-NRs was prepared by reaction with ammonia and subsequent hydrothermal growth. Its properties were characterized by X-ray diffraction, Raman spectroscopy and scanning electron microscopy. Subsequently, thiol-modified aptamers were self-assembled on AuNPs that had been electrodeposited on the surface of the modified electrode. The presence of ZnO-NRs on the electrode increases the loading with AuNPs and aptamers. It also warrants a relatively stable microenvironment for the aptamers. In the presence of PAT, it will form a complex with the aptamer on the electrode surface. This hinders electron transfer from the electrode to the redox probe hexacyanoferrate and results in reduced current, which is typically measured at 0.176 V (vs. Ag/AgCl). The concentration of PAT can be calculated from the differences in the peak current before and after incubation with PAT. The assay has a linear response in the 50 ng·mL−1 to 0.5 pg·mL−1 PAT concentration range and a 0.27 pg·mL−1 lower detection limit. The sensor is specific, reproducible, repeatable, and long-term stable. It was successfully applied to the determination of PAT in spiked juice samples.

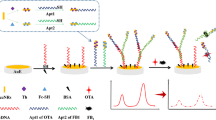
Schematic representation of aptamer based detection of patulin (PAT). It is based on the fact that ZnO nanorods on the surface of the electrode increase the loading of the gold nanoparticles and the aptamers, thereby improving the electrode performance.






Similar content being viewed by others
References
Zhang WG, Han Y, Chen XM, Luo XL, Wang JL, Yue TL, Li ZH (2017) Surface molecularly imprinted polymer capped Mn-doped ZnS quantum dots as a phosphorescent nanosensor for detecting patulin in apple juice. Food Chem 232:145–154
Norstadt FA, Mccalla TM (1963) Phytotoxic substance from a species of penicillium. Sci 140(3565):410–411
Yuan XU, Jiang LP, Chen M, Geng CY, Wei YW, Yang G, Zhang LF, Liu XF (2014) The lysosomal pathway of dna damage induced by patulin in hek-293 cells. J Toxicol 28(3):203–207
dos Santos ID, Pizzutti IR, Dias JV, Fontana MEZ, Brackmann A, Anese RO, Thewes FR, Marques LN, Cardoso CD (2018) Patulin accumulation in apples under dynamic controlled atmosphere storage. Food Chem 255:275–281
Welke JE, Hoeltz M, Dottori HA, Noll IB (2009) Quantitative analysis of patulin in apple juice by thin-layer chromatography using charge coupled device detector. Food Addit Contam 26(5):754–758
Liu BJ, Peng XN, Meng XH (2018) Effective biodegradation of mycotoxin patulin by porcine pancreatic lipase. Front Microbiol 9(615):1–7
Tarter EJ, Scott PM (1991) Determination of patulin by capillary gas chromatography of the heptafluorobutyrate derivative. J Chromatogr 538(2):441–446
Wang Y, Shan TT, Yuan YH, Zhang ZW, Guo CF, Yue TL (2017) Evaluation of penicillium expansum for growth, patulin accumulation, nonvolatile compounds and volatile profile in kiwi juices of different cultivars. Food Chem 228:211–218
Rodríguez A, Luque MI, Andrade MJ, Rodríguez M, Asensio MA, Córdoba JJ (2011) Development of real-time PCR methods to quantify patulin-producing molds in food products. Food Microbiol 28(6):1190–1199
Tsao R, Zhou T (2000) Micellar electrokinetic capillary electrophoresis for rapid analysis of patulin in apple cider. J Agric Food Chem 48(11):5231–5235
Sang F, Liu J, Zhang X, Pan JX (2018) An aptamer-based colorimetric Pt(II) assay based on the use of gold nanoparticles and a cationic polymer. Microchim Acta 185(5):267–274
Wei G, Pi FW, Zhang HX, Sun JD, Zhang YZ, Sun XL (2017) A novel molecularly imprinted electrochemical sensor modified with carbon dots, chitosan, gold nanoparticles for the determination of patulin. Biosens Bioelectron 98:299–304
Chen YX, Wu X, Huang KJ (2018) A sandwich-type electrochemical biosensing platform for microrna-21 detection using carbon sphere-Mos 2, and catalyzed hairpin assembly for signal amplification. Sensor Actuat B-Chiem 270:179–186
Azadbakht A, Roushani M, Abbasi AR, Menati S, Derikvand Z (2016) A label-free aptasensor based on polyethyleneimine wrapped carbon nanotubes in situ formed gold nanoparticles as signal probe for highly sensitive detection of dopamine. Mat Sci Eng C-Mater 68:585–593
Liu RJ, Wu H, Lv L, Kang XJ, Cui CB, Feng J, Guo ZJ (2018) Fluorometric aptamer based assay for ochratoxin a based on the use of exonuclease III. Microchim Acta 185(5):254–259
Chen YX, Huang KJ, He LL, Wang YH (2017) Tetrahedral dna probe coupling with hybridization chain reaction for competitive thrombin aptasensor. Biosens Bioelectron 100:274–281
He BS, Du GA (2018) Novel electrochemical aptasensor for ultrasensitive detection of sulfadimidine based on covalently linked multi-walled carbon nanotubes and in situ synthesized gold nanoparticle composites. Anal Bioanal Chem 410(12):2901–2910
Wang YH, Huang KJ, Wu X (2018) Recent advances in transition-metal dichalcogenides based electrochemical biosensors: a review. Biosens Bioelectron 97:305–316
Bhattacharya M, Hong S, Lee D, Cui T, Goyal SM (2010) Carbon nanotube based sensors for the detection of viruses. Sensors Actuat B-Chem 155(1):67–74
Chen YX, Huang KJ, Niu KX (2018) Recent advances in signal amplification strategy based on oligonucleotide and nanomaterials for microRNA detection-a review. Biosens Bioelectron 99:612–624
Heydari-Bafrooei E, Shamszadeh NS (2017) Electrochemical bioassay development for ultrasensitive aptasensing of prostate specific antigen. Biosens Bioelectron 91:284–292
Amouzadeh MT, Shamsipur M, Saber R, Sarkar S (2017) Flow injection amperometric sandwich-type aptasensor for the determination of human leukemic lymphoblast cancer cells using mwcnts-pdnano/ptca/aptamer as labeled aptamer for the signal amplification. Anal Chim Acta 985:61–68
Zhao CZ, Liu L, Ge JY, He YY (2017) Ultrasensitive determination for flavin coenzyme by using a ZnO nanorod photoelectrode in a four-electrode system. Microchim Acta 184(7):2333–2339
Wei YY, Li Y, Liu XQ, Xian YZ, Shi GY, Jin LT (2011) Zno nanorods/au hybrid nanocomposites for glucose biosensor. Biosens Bioelectron 26(1):275–278
Heydari-Bafrooei E, Amini M, Ardakani MH (2016) An electrochemical aptasensor based on tio 2 /mwcnt and a novel synthesized schiff base nanocomposite for the ultrasensitive detection of thrombin. Biosens Bioelectron 85:828–836
Fang LX, Huang KJ, Zhang BL, Liu YJ, Zhang QY (2014) A label-free electrochemistry biosensor based flower-like 3-dimensional zno superstructures for detection of dna arrays. New J Chem 38(12):5918–5924
Huang KJ, Sun JY, Xu CX, Niu DJ, Xie WZ (2010) A disposable immunosensor based on gold colloid modified chitosan nanoparticles-entrapped carbon paste electrode. Microchim Acta 168(1–2):51–58
Liu XP, Deng YJ, Jin XY, Chen LG, Jiang JH, Shen GL, Yu RQ (2009) Ultrasensitive electrochemical immunosensor for ochratoxin a using gold colloid-mediated hapten immobilization. Anal Biochem 389(1):63–68
Wu SJ, Duan N, Zhang WX, Zhao S, Wang ZP (2016) Screening and development of dna aptamers as capture probes for colorimetric detection of patulin. Anal Biochem 508:58–64
Jia W, Dang SH, Liu HR, Zhang ZX, Yu CY, Liu XG, Xu BS (2012) Evidence of the formation mechanism of zno in aqueous solution. Mater Lett 82(4):99–101
Yuan BQ, Zhang JC, Zhang RC, Shi HZ, Wang N, Li JW, Ma FJ, Zhang DJ (2016) Cu-based metal–organic framework as a novel sensing platform for the enhanced electro-oxidation of nitrite. Sensors Actuat B-Chem 222:632–637
Duan N, Zhang WX, Wu SJ, Wang ZP (2016) A colorimetric method for Patulin detection based on aptamer and gold nanoparticles. Sci Sinica Chim 46(3):268–273
Fang GZ, Wang H, Yang YK, Liu GY, Wang S (2016) Development and application of a quartz crystal microbalance sensor based on molecularly imprinted sol-gel polymer for rapid detection of patulin in foods. Sensors Actuat B-Chem 237:239–246
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 61301037), the Cultivation Plan for Young Core Teachers in Universities of Henan Province (No. 2017GGJS072), the Henan Science and Technology Cooperation Project (Grant No. 172106000014), and the Youth Backbone Teacher Training Program of Henan University of Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 761 kb)
Rights and permissions
About this article
Cite this article
He, B., Dong, X. Aptamer based voltammetric patulin assay based on the use of ZnO nanorods. Microchim Acta 185, 462 (2018). https://doi.org/10.1007/s00604-018-3006-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3006-0