Abstract
Purpose
Perioperative surgical stress and systemic inflammation resulting from complex interactions between cancer and the host play an important role in cancer progression. This retrospective study compared the prognostic impact of various perioperative cumulative inflammation- and nutrition-based markers in patients with gastric cancer (GC).
Methods
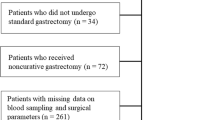
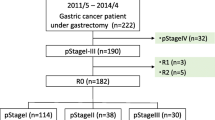
This study included 301 patients with a histopathological diagnosis of gastric adenocarcinoma who underwent curative surgery. Perioperative cumulative markers were calculated using the newly developed trapezoidal area method.
Results
The cumulative prognostic nutritional index (cum-PNI) had the highest area under the receiver operating characteristic (ROC) curve for predicting the overall survival (OS) as well as the relapse-free survival (RFS). The cum-PNI was significantly correlated with tumor-related factors, including tumor size, depth of invasion, lymph node metastasis, lymphatic involvement, vascular involvement, and TNM stage classification. The cum-PNI was also significantly correlated with surgical factors, including surgical approach, gastrectomy, lymphadenectomy, intraoperative blood loss, and postoperative complications. Furthermore, the OS and RFS were poorer in patients with a low cum-PNI (< 236.3) than in those with a high cum-PNI (> 236.3). A multivariate analysis indicated that a low cum-PNI was an independent prognostic indicator in patients with GC.
Conclusions
The cum-PNI might be useful for predicting the prognosis and guiding the perioperative management of patients with GC.





Similar content being viewed by others
Data availability
All authors are available for the data of this study.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1–27.
Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–63.
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99.
McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223–6.
Shimada H, Takiguchi N, Kainuma O, Soda H, Ikeda A, Cho A, et al. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 2010;13:170–6.
Jiang X, Hiki N, Nunobe S, Kumagai K, Kubota T, Aikou S, et al. Prognostic importance of the inflammation-based Glasgow prognostic score in patients with gastric cancer. Br J Cancer. 2012;107:275–9.
Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40:440–3.
Miyatani K, Saito H, Kono Y, Murakami Y, Kuroda H, Matsunaga T, et al. Combined analysis of the pre- and postoperative neutrophil-lymphocyte ratio predicts the outcomes of patients with gastric cancer. Surg Today. 2018;48:300–7.
Murakami Y, Saito H, Kono Y, Shishido Y, Kuroda H, Matsunaga T, et al. Combined analysis of the preoperative and postoperative prognostic nutritional index offers a precise predictor of the prognosis of patients with gastric cancer. Surg Today. 2018;48:395–403.
Kono Y, Saito H, Murakami Y, Shishido Y, Kuroda H, Matsunaga T, et al. Postoperative ratio of the maximum C-reactive protein level to the minimum peripheral lymphocyte count as a prognostic indicator for gastric cancer patients. Surg Today. 2019;49:206–13.
Okugawa Y, Toiyama Y, Fujikawa H, Kawamura M, Yasuda H, Yokoe T, et al. Cumulative perioperative lymphocyte/C-reactive protein ratio as a predictor of the long-term outcomes of patients with colorectal cancer. Surg Today. 2021.
The 15th edition (3rd English edition) of the Japanese classification of gastric carcinoma.
Feng JF, Huang Y, Liu JS. Combination of neutrophil lymphocyte ratio and platelet lymphocyte ratio is a useful predictor of postoperative survival in patients with esophageal squamous cell carcinoma. Onco Targets Ther. 2013;6:1605–12.
Shishido Y, Saito H, Shimizu S, Kono Y, Murakami Y, Miyatani K, et al. Prognostic significance of platelet × C-reactive protein multiplier in patients with esophageal squamous cell carcinoma. Surg Today. 2020;50:185–92.
Yue L, Lu Y, Li Y, Wang Y. Prognostic value of C-reactive protein to albumin ratio in gastric cancer: a meta-analysis. Nutr Cancer. 2021;73:1864–71.
Song GM, Tian X, Liang H, Yi LJ, Zhou JG, Zeng Z, et al. Role of enteral immunonutrition in patients undergoing surgery for gastric cancer: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2015;94: e1311.
Ryan AM, Reynolds JV, Healy L, Byrne M, Moore J, Brannelly N, et al. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double-blinded randomized controlled trial. Ann Surg. 2009;249:355–63.
Shiromizu A, Suematsu T, Yamaguchi K, Shiraishi N, Adachi Y, Kitano S. Effect of laparotomy and laparoscopy on the establishment of lung metastasis in a murine model. Surgery. 2000;128:799–805.
Xia X, Zhang Z, Zhu C, Ni B, Wang S, Yang S, et al. Neutrophil extracellular traps promote metastasis in gastric cancer patients with postoperative abdominal infectious complications. Nat Commun. 2022;13:1017.
Onodera T, Goseki N. Kosaki G [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. 1984;85:1001–5.
The Japanese Gastric Cancer Treatment Guidelines 2014 (version 4).
Gao Y, Zhou S, Jiang W, Huang M, Dai X. Effects of ganopoly (a Ganoderma lucidum polysaccharide extract) on the immune functions in advanced-stage cancer patients. Immunol Invest. 2003;32:201–15.
Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98:268–74.
Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–41.
Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27.
Yamada T, Hayashi T, Cho H, Yoshikawa T, Taniguchi H, Fukushima R, et al. Usefulness of enhanced recovery after surgery protocol as compared with conventional perioperative care in gastric surgery. Gastric Cancer. 2012;15:34–41.
Tian YL, Cao SG, Liu XD, Li ZQ, Liu G, Zhang XQ, et al. Short- and long-term outcomes associated with enhanced recovery after surgery protocol vs conventional management in patients undergoing laparoscopic gastrectomy. World J Gastroenterol. 2020;26:5646–60.
Okugawa Y, Toiyama Y, Fujikawa H, Kawamura M, Yasuda H, Yokoe T, et al. Cumulative perioperative lymphocyte/C-reactive protein ratio as a predictor of the long-term outcomes of patients with colorectal cancer. Surg Today. 2021;51:1906–17.
Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11:8–13.
Jensen MB, Nørager CB, Sommer T, Madsen MR, Laurberg S. Changes in fatigue and physical function following laparoscopic colonic surgery. Pol Przegl Chir. 2014;86:82–8.
Bae JM, Park JW, Yang HK, Kim JP. Nutritional status of gastric cancer patients after total gastrectomy. World J Surg. 1998;22:254–60.
Aoyama T, Sato T, Maezawa Y, Kano K, Hayashi T, Yamada T, et al. Postoperative weight loss leads to poor survival through poor S-1 efficacy in patients with stage II/III gastric cancer. Int J Clin Oncol. 2017;22:476–83.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Funding
The authors have no financial support to declare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional review board/ethics committee, the national research committee, and the 1964 Declaration of Helsinki and its later amendments. The Institutional Review Board of Tottori University Hospital approved the study (approval no. 20A243) and waived the requirement for informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miyatani, K., Sakano, Y., Makinoya, M. et al. A low cumulative perioperative prognostic nutritional index predicts poor long-term outcomes in patients with gastric cancer: A single-center retrospective study in Japan. Surg Today 53, 1294–1304 (2023). https://doi.org/10.1007/s00595-023-02688-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-023-02688-8