Abstract
Purpose
To describe the physiology of spinal growth in patients with adolescent idiopathic scoliosis (AIS).
Methods
Narrative review of the literature with a focus on mechanisms of growth.
Results
In his landmark publication On Growth and Form, D’Arcy Thompson wrote that the anatomy of an organism reflects the forces it is subjected to. This means that mechanical forces underlie the shape of tissues, organs and organisms, whether healthy or diseased. AIS is called idiopathic because the underlying cause of the deformation is unknown, although many factors are associated. Eventually, however, any deformity is due to mechanical forces. It has long been shown that the typical curvature and rotation of the scoliotic spine could result from vertebrae and intervertebral discs growing faster than the ligaments attached to them. This raises the question why in AIS the ligaments do not keep up with the speed of spinal growth. The spine of an AIS patient deviates from healthy spines in various ways. Growth is later but faster, resulting in higher vertebrae and intervertebral discs. Vertebral bone density is lower, which suggests less spinal compression. This also preserves the notochordal cells and the swelling pressure in the nucleus pulposus. Less spinal compression is due to limited muscular activity, and low muscle mass indeed underlies the lower body mass index (BMI) in AIS patients. Thus, AIS spines grow faster because there is less spinal compression that counteracts the force of growth (Hueter–Volkmann Law). Ligaments consist of collagen fibres that grow by tension, fibrillar sliding and the remodelling of cross-links. Growth and remodelling are enhanced by dynamic loading and by hormones like estrogen. However, they are opposed by static loading.
Conclusion
Increased spinal elongation and reduced ligamental growth result in differential strain and a vicious circle of scoliotic deformation. Recognising the physical and biological cues that contribute to differential growth allows earlier diagnosis of AIS and prevention in children at risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
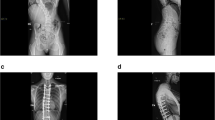
Adolescent idiopathic scoliosis (AIS) is a slow, three-dimensional deformation of the spine, resulting in bending and twisting of the vertebral column (Fig. 1). The cause of AIS is unknown (hence idiopathic) and likely multifactorial, including genetic, epigenetic, neural, endocrinological and environmental aspects (for excellent reviews on etiopathological theories of AIS, see [1,2,3,4]). Nevertheless, it is a law of physics that any deformation of a structure is due to a mechanical force. Thus, the first question in the etio-pathogenesis of AIS should be: Which force drives the scoliotic deformation of the spine? In AIS, more than in other types of scoliosis, growth seems to be a necessary condition, not only because AIS coincides with the adolescent growth spurt [5], but also because faster growth results in larger Cobb angles [6], and curve progression slows substantially at skeletal maturity [7]. It has long been recognised, and explicitly pointed out by D’Arcy Thompson, that skeletal growth is not only a biological process, but also a physical force [8]. In the growth plates, chondrocytes become hypertrophic as they increase their volume up to 15-fold by the osmotic imbibition of water [9]. In the extracellular matrix of cartilaginous tissues like growth plates and intervertebral discs, a high negative charge density of glycosaminoglycans provides hydrophilicity and thereby high swelling pressure [10]. Skeletal growth thus is a force created by the osmotic pressure in the cells of the growth plates and the intervertebral discs (pressure × area = force). Intervertebral discs of AIS patients are higher than those of age-matched controls [11, 12] and intradiscal pressure is increased as well [13]. Vertebral bodies also grow longer in AIS patients [11, 14], which is indicative for more active growth plates. That growth is a force is further illustrated by the notion that it can be modulated by physical stress: Hueter [15] and Richard von Volkmann [16] observed that the growth of bones is inhibited by compression on the growth plates and enhanced by tension, a phenomenon now known as the Hueter–Volkmann Law [17].
While skeletal growth may be seen as the driving force of AIS, excessive growth alone does not explain the bending and twisting of the scoliotic spine. For that, the spine must be tethered by a stiff material that is tightly attached to the expanding structure. Somerville already showed in 1952 that a failure of growth in the posterior elements leads to a local lordosis and subsequently to rotation of the vertebrae and lateral flexion of the spine [18]. This concept of differential growth has since been confirmed by others using a variety of models [19,20,21,22,23]. Most recently, Crijns et al. [23] created a physical model of the growing spine and showed that differential growth first induces hypokyphosis and mild lateral bending, and then suddenly escalates into a scoliotic deformity, consistent with clinical observations of AIS in growing adolescents. Vertebral rotations were not reported, but clearly observed with the posterior elements pointing to the midline, also in line with clinical observations. Notably, the growth difference in this model is only 30 mm for the entire spine, a strain of some 5%. The authors also highlight the role of ventral structures, like the abdominal muscles and the linea alba that connect the sternum to the ilium [23]; these appear to be necessary to obtain a scoliotic deformity rather than a simple extension without a twist (see also Roaf [19]). Overall, these models support the idea that AIS results from a subtle growth imbalance between tissues, rather than a left–right asymmetry [22, 24]. However, the models do not explain why the ligaments do not match the growth of the vertebrae and intervertebral discs in young adolescents, or why this happens much more frequently in girls. That question is addressed here.
AIS is a multifactorial disease, including genetic and epigenetic factors, hormones, metabolism and more [1,2,3,4]. The purpose of this perspective paper is to describe the physiology of spinal growth, more specifically the growth of vertebrae and intervertebral discs on the one hand and of spinal ligaments on the other hand. Together, they define the mechanobiological mechanism underlying scoliotic deformations of the spine. Physical and biological cues that affect spinal growth, like genes, hormones or muscular loading, may be identified as risk factors and will be integrated into a new conceptual framework. Understanding the physiology of differential growth may lead to new strategies for prevention and early intervention of AIS in young adolescents.
Spinal growth
The entire spine
The natural growth of the healthy human spine is abundantly described in literature [25,26,27]. On average, it declines from more than 12 cm/year in the first year of life to about 2.3 cm/year from age 6 until puberty. Girls have a growth spurt at age 12–13 and boys at age 14–15, with stronger spinal growth in the second year of puberty. After that, there is a decline to zero at adulthood (data from Stücker [26]). The spine mainly grows in the vertebral bodies, lumbar more than thoracic more than cervical [27]. The cervical and thoracic intervertebral discs only grow slightly in the first four years, after which disc height stabilizes. Lumbar discs grow somewhat faster until about the age of 7 and also show a small growth spurt at the start of puberty [27]. With AIS, growth looks a bit different (Fig. 2). Yim et al. [6] studied growth of 611 Asian girls with AIS and 296 age-matched controls between the age of 12 and 16. The AIS girls were divided between moderate (Cobb angle 20°–40°) and severe scoliosis (more than 40°). In a cross-sectional analysis, the girls with severe scoliosis were significantly shorter than controls at the age of 12, but significantly taller at the age of 15 (Fig. 2). The girls with mild scoliosis were as tall as controls at the age of 12, but as tall as severe scoliotic girls at the age of 15. Consequently, the growth rate was much higher in girls with severe AIS (3.6 cm/year) than in girls with moderate AIS (2.3 cm/year) or healthy controls (1.6 cm/year) (Fig. 2). The authors also provide longitudinal data of girls developing AIS, which show the same trend [6]. The later onset of growth in girls with severe AIS may be related to a significantly later menarche (5.9 months compared to controls) that was also observed [6]. That late menarche correlates with AIS has also been reported by others [28,29,30], but how this leads to a mechanical deformation remains as yet unexplained.
Growth of AIS patients is later but faster than age-matched controls. More spinal growth leads to larger Cobbs curves. Data underlying graph: Yim et al. [6]. Controls: girls with Cobbs angle less than 20°. AIS20: patients with Cobb angle between 20° and 40°. AIS40: patients with Cobbs angle more than 40°
The intervertebral discs
In recent years, multiple studies showed that intervertebral discs are higher in AIS patients than in healthy age-matched controls. Chen et al. [31] found 10–20% higher intervertebral discs at all thoracic levels of the spine, and Ponrartana et al. [11] described an increase in lumbar discs of some 20% in both boys and girls with AIS. Brink and colleagues also found that the difference in spinal growth between AIS patients and controls is mainly due to the intervertebral discs [12]. In principle, there are two mechanisms that cause increased intervertebral disc height: reduced spinal compression [32] and higher intradiscal pressure [33, 34]. These mechanisms seem to exclude each other, since disc height is reported to be inversely related to disc pressure [35], i.e.: reduced axial compression relaxes the disc, increases disc height and reduces intradiscal pressure [34]. Nevertheless, despite the reported increase in disc height [11, 12, 31], Meir et al. measured an intradiscal pressure of about 0.25 MPa in anaesthesized AIS patients in supine position [13, 36]. This is 70–100% more than in age-matched, not anaesthesized controls (0.12–0.15 MPa) [36]. Increased disc pressure in AIS patients may be related to the continued presence of notochordal cells in adult intervertebral discs [37], which have large vacuoles with a high concentration of hydrophilic solutes that elevate osmotic pressure [38, 39]. Also, notochordal cells induce and stimulate matrix synthesis in the intervertebral disc [40, 41], which may further increase static pressure and disc height.
Normally, notochordal cells vanish at the onset of puberty [40, 42] and this appears to be related to dynamic loading, presumably as a result of the activities of daily life. In an ex-vivo study using porcine intervertebral discs, Li et al. [43] showed that the number and vitality of notochordal cells decrease with higher amplitude and frequency of dynamic compression. Guehring and colleagues obtained similar results in an in vivo rabbit model [44]. Thus, that notochordal cells normally disappear at the onset of puberty may be due to the increased amplitude and frequency of dynamic loading during the activities of daily life, as well as higher muscular force and body mass. The loss of notochordal cells then results in a decrease of intradiscal pressure [45]. Reversely, the continued presence of notochordal cells in adult AIS patients suggests that the intradiscal pressure is high and that the dynamic loading of the spine was insufficient to eliminate the notochordal cells.
The vertebral bodies
Not only the intervertebral discs, but also the vertebral bodies are higher in AIS patients than in healthy controls. Guo et al. [14] report consistently higher vertebral body height at thoracic levels, while Ponrartana et al. [11] found the same at lumbar levels. Following the Law of Hueter–Volkmann [17], these data suggest that the vertebrae are subjected to a reduced spinal compression, thereby allowing enhanced longitudinal growth. At the same time, the cross-sectional areas of the vertebrae appear to be smaller, resulting in more slender vertebrae in AIS patients [11]. A smaller cross-sectional area of the vertebrae is also indicative for a reduced spinal compression, because bones in general grow more stout under increased loading to maintain equal stress [46]. Thus, spines in AIS patients are more slender than in age-matched controls. This theoretically creates a higher risk of Euler buckling, but Euler buckling is not considered the mechanism underlying AIS, because the spine is not an elastic rod [47] but rather a chain of vertebrae hinged by flexible intervertebral discs [48, 49]. Also, the deformation is not instantaneous, but rather slow and related to the growing spine [50]. Yet, increased vertebral growth contributes to differential growth, which underlies the scoliotic deformation of the spine [18, 19, 50, 51].
Spinal loading
Vertebral bodies
Along with vertebral morphology, vertebral bone mineral density has been assessed in AIS patients. Bone mineral density is a strong indicator of spinal loading since it highly correlates with muscle mass (r = 0.92) in a population of children, women and men [52]. This implies that bone mineral density is a strong indicator of muscular activity. Cook et al. [53] already reported a significantly lower bone mineral density in AIS patients, 15% less than in healthy controls. Lee and colleagues also reported lower bone mineral density in AIS patients as compared to age-matched controls, and more so in patients with higher Cobb angles [54]. More recently, Ramos et al. [55] performed an MRI study in which they observed a vertebral bone quality (VBQ) score of 2.5 in AIS patients, compared to 2.1 in age-matched controls, also indicating poorer bone quality. These studies all support the notion that the spines of AIS patients are subjected to less dynamic compression than the spines of age-matched controls. This fits with the observations of higher growth rates and increased intervertebral disc height discussed above. Also, the lower bone mineral density in the thoracic spine [54] may be indicative for a lower spinal compression and higher risk of scoliotic deformations than the lumbar or cervical levels.
Muscle mass
Faster growth, higher intervertebral discs, more slender vertebral bodies and low vertebral bone density are all indications that spinal compression is reduced in AIS patients. This compression mainly results from gravity and muscular activity [50]. Barrios and colleagues report that girls with AIS have a progressive decrease of BMI and more general a change in body composition (somatotype) [56]. In their large study on Asian girls, Yim and colleagues also reported body weight and body mass index (BMI) as a function of age [6]. Interestingly, girls that develop mild AIS weigh less at age 12 than healthy controls and more than girls with severe AIS. At age 15, however, the average weight of the three groups is more or less the same. Related to body height, however, there is a considerable difference between AIS patients and healthy controls: BMI is 17 or less in AIS patients at age 12 and more than 18.5 in healthy controls; through the years the difference remains more than one unit (Fig. 3). It is further interesting to note that the absolute BMI of the AIS patients is close to 17.5, the definition of anorexia nervosa in adults [57]. Patients with anorexia and other eating disorders are indeed at much greater risk to develop scoliosis than healthy controls [58].
Body mass index in Asian girls as a function of age. AIS patients have substantially lower body mass index than healthy controls, with values comparable to patients with anorexia nervosa (17.5 kg/m2). Data underlying graph: Yim et al. [6]. Controls: girls with Cobbs angle less than 20°. AIS20: patients with Cobb angle between 20° and 40°. AIS40: patients with Cobbs angle more than 40°
Miyagi et al. [59] reported the lean mass index (LMI) of AIS patients and healthy controls, essentially the amount of muscle mass in relation to body height and as such a measure of muscularity. As expected, LMI is lower for AIS patients at all ages between 10 and 17. Further, LMI, BMI and bone mass all declined with increasing Cobb angle, while fat mass did not. This implies that loss of weight is mainly due to loss of muscle mass, which also results in lower bone mass. Starcevic–Klasan and colleagues reported that also muscular power is reduced in AIS patients: they matched AIS patients (n = 93) and healthy controls (n = 90) for age, weight, height and BMI, and found that iliopsoas strength was reduced by more than 10% [60]. Shahidi et al. [61] report that muscles on either side of the apex are atrophic compared to non-scoliotic individuals, and demonstrate levels of collagen similar to severe degenerative spinal pathologies. These observations all support the notion that muscular activity is reduced in AIS patients, not only by muscular mass but also by muscular strength. It leads to less spinal compression, lower bone mass, lower BMI, and accelerated growth of vertebrae and intervertebral discs [50].
Ligamental growth
The structure of the ligament
While bone and cartilage grow by osmotic pressure of cells and matrix, tendons, ligaments, muscles, nerves and other tissues need external tension in order to grow. This way, the tensegrity and mechanical functionality of the musculoskeletal system remain intact during growth [50]. Ligaments, tendons and other fasciae essentially consist of parallel, discontinuous collagen fibrils that are interconnected by a non-collagenous matrix (Fig. 4a) [62]. This non-collagenous matrix consists of glycoproteins like proteoglycans, but also lubricin that allows sliding and elastin that provides elasticity (for a detailed description of tendon and ligament composition and architecture, see Thorpe et al. [63]). The majority of proteoglycans are small, leucine-rich proteoglycans (SLRPs), of which decorin is the most abundant one (80%) [62]. Decorins are known to inhibit the lateral fusion of collagen fibrils [64] by binding non-covalently to specific amino acids of the collagen fibrils. Glycosaminoglycans are able to connect to decorins on adjacent collagen fibrils to form an interfibrillar bridge, thereby securing mechanical integrity under tension (Fig. 4b) [65].
The structure and growth of tendons and ligaments. These tissues essentially exist of discontinuous, parallel collagen fibres interconnected by a collagenous interfibrillar matrix (a). Connections are made by small, leucine-rich proteoglycans (SLRPs), in particular decorins, that are interconnected by larger proteins like glycosaminoglycans (b). For growth, tension is required and breaking of the cross-links, which are then re-attached on a different location (c)
Growth by fibrillar sliding
Mechanical stiffness and strength of tendons and ligaments are related to the cross-link density of the collagen fibrils involved: the more cross-links, the stiffer and stronger the ligament. In order to allow growth, tension must apply and the interfibrillar proteins must break, so that collagen fibrils can slide along each other longitudinally, and then reconnect in order to restore mechanical integrity (Fig. 4c) [66]. The collagen fibrils are then replenished by cell-created procollagens to fill the gaps and restore mechanical integrity (Fig. 4c). The breaking of interfibrillar bridges is essentially microdamage induced by dynamic mechanical loading [67], presumably by the activities of daily life. Thus, the growth of ligaments, tendons and other fasciae is a process of continuous remodelling under dynamic longitudinal tension. In adult tendons and ligaments, the cross-linking density is so high that fibrillar sliding is no longer possible [68]. In AIS, ligamentous growth appears to be inhibited and one reason may be the lack of dynamic loading that is required to break the cross-links and allow fibrillar sliding of the collagens and ligamental growth. Another reason may be that hormones affect the physiology and mechanical properties of ligaments; this is discussed in “A role for hormones” section.
Static strain
Dynamic loading thus induces micro-damage to tendons and ligaments and thereby facilitate remodelling and growth. Static tension, on the other hand is reported to suppress the turnover and degradation of connective tissues [69, 70]. Saini et al. [71] review this phenomenon in detail and ascribe this effect to the non-conformity of enzymes to cleavage sites along the collagen fibrils, that is: matrix metalloproteinases and collagenases are no longer able to reach and digest the catalytic domains along the collagen fibrils. Also, stretched tendons and ligaments contract perpendicularly due to the mechanical Poisson effect and thereby decrease the permeability of the matrix for the interstitial transport of enzymes. Similarly, collagen degradation was reported to be inhibited by collagen cross-linking, as in matured organisms [68]. Thus, the condensation of the extracellular matrix decreases the availability and efficacy of enzymes and thereby reduce the growth potential of tendons and ligaments. On the other hand, dynamic loading enhances the transport of degrading enzymes through the matrix [72] and thereby support remodelling and growth.
Integration of data: a conceptual framework for the etiology of AIS
Adolescent idiopathic scoliosis is a deformation of the spine related to growth. The literature discussed above shows that vertebral bodies and intervertebral discs of AIS patients grow faster than of healthy, age-matched controls. If ligaments cannot keep pace with this growth, a scoliotic-like deformation of the spine is inevitable [18, 19, 23, 24, 73]. Indeed, enhanced growth rate of the spine is not sufficient to induce AIS, it also requires retarded ligamentous longitudinal growth. A physical model of this phenomenon shows that it only requires subtle differential growth (30 mm along the whole thoracolumbar spine, some 5%; [23]) to induce a scoliotic deformation. It also shows that for most of the differential growth (20–25 mm) the scoliosis remains low (Cobbs angle less than 10°), after which the spine suddenly warps out, in line with clinical observations. In Fig. 5 the growth of the spine and the attached ligaments are integrated into a conceptual framework for the scoliotic deformation occurring in AIS.
It appears from literature that decreased muscular loading plays a dominant role in the growth of both, the spine (red in Fig. 5) and the ligaments (blue), in opposite directions. While healthy dynamic loading brings the notochordal cells in the nucleus pulposus in a state of apoptosis [43,44,45], reduced loading in AIS patients preserves these cells [37], which then stimulate the production of the hydrophilic matrix [74], resulting in higher intradiscal pressure and growth [75]. At the same time, lower spinal compression facilitates axial vertebral growth according to the Hueter–Volkmann Law, leading to higher vertebral bodies as well.
Ligamental growth not only requires static longitudinal tension due to the growth of vertebrae and intervertebral discs, but also dynamic loading that breaks the cross-links between collagen fibrils, which are then able to slide along each other to allow growth [66]. Thus, a lack of dynamic loading, presumably due to decreased muscular strength and physical activity, slows down ligamental remodelling and growth. This effect is aggravated by static loading, which condenses the ligaments and inhibits the enzymatic breakdown of the matrix [71]. This increased static loading of the spinal ligaments seems to originate in the increased pressure in the intervertebral discs due to the presence of notochordal cells that also produce highly-hydrophilic extracellular matrix. This then further increases intradiscal pressure and intervertebral disc height and leads to AIS in a vicious circle.
Discussion
Adolescent idiopathioc scoliosis is an enigmatic deformation of the spine with unknown cause (hence idiopathic). Its origin has been studied for decades and over the years many factors have been incriminated, including genes, hormones, metabolism, the nervous system and epigenetics [1,2,3,4]. Most of these factors show an association with AIS, for example in genome-wide association studies (GWAS) [2, 76, 77]. Associations, however, describe a statistical probability, not a mode of action. Indeed, it is not always clear how genes like PAX1 or LBX1 [2] or hormones like melatonin [78] contribute to the mechanical deformation of the spine (i.e.: their mechanical phenotype). Thus, while association studies are extremely useful in understanding the role of certain genes or homones in AIS, they do not explain the mechanics underlying the deformity. The hormone relaxin, on the other hand, has been shown to inhibit cross-linking of collagens in ligaments [79], which decreases mechanical stiffness of the ligament and thereby enhances growth capacity. The mechanical phenotypes of melatonin, PAX1 or LBX1 are less straight-forward and currently can only be qualified as risk factors for AIS.
The risk of reduced muscularity to develop scoliosis
The conceptual framework presented here provides a mechanistic, mechanobiological explanation for the differential growth of the spinal column and the ligaments attached to it, resulting in the characteristic scoliotic deformity of the spine. It appears that reduced muscularity is a major risk factor for the development of AIS, as it has an effect on the growth of both, the spine and the ligaments, but in opposite directions, resulting in differential growth. The concept does not tell, however, what underlies a reduced muscular strength in the first place. Indeed, there may be multiple causes for that, like a neuromuscular disease, a genetic mutation, a brain disorder (e.g. of the cerebellum or the pineal gland), an eating disorder, or simply disuse, all of which have been suggested as an underlying cause of scoliosis [1,2,3,4]. In this context, one may wonder whether the classical differentiation between neuromuscular scoliosis (e.g. from Duchenne muscular distrophy) and adolescent idiopathic scoliosis is really that different, except that in the case of muscular dystrophy the cause is obvious and in AIS it is more subtle and appears later in life. Brink and colleagues already pointed this out in a direct anatomical comparison between patients with neuromuscular and adolescent scoliosis [12]. The conceptual framework presented in Fig. 5 suggests that differential growth underlies all types of juvenile and adolescent scoliosis.
A role for hormones
As explained in the introduction, skeletal growth is a mechanical force, but it is also subjected to a large variety of hormones [80]. Some of these directly affect growth (like growth hormone produced by the pituary gland and IGF-1 produced by the liver), and it appears that e.g. integrated growth hormone levels are substantially and significantly higher in AIS patients under 12, but not different at higher age [80, 81]. Other hormones are related to brain function (growth hormone releasing hormone (GHRH) produced by the hypothalamus) or nutrition (insulin produced by the pancreas; hunger hormones ghrelin and leptin, produced by the stomach and fat cells, respectively) [82]. Nutrition presumably determines the growth and strengthening of muscles, as suggested by the higher incidence of scoliosis in patients with eating disorders [58]. Melatonin (produced by the pineal gland) is widely investigated in the context of scoliosis, because experimental rat studies show that a depletion is related to the development of scoliosis [78] and that the volume of the pineal gland is smaller in AIS patients than in healthy controls [83]. Nevertheless, its mechanism remains obscure [84,85,86]. An interesting cue may be that a high concentration of melatonin receptors is found in the cerebellum [87], the small brain that governs motor control and balance [88] and thus may underlie muscular activity. Another interesting hormone is relaxin, a hormone produced by the prostate in men and in breasts and ovary in women [89]. Relaxin appears to interfere with connective tissues, presumably as a competitor of SLRPs like decorin that function as anchor points for cross-links between collagen fibrils [79]. Considering the late menarche in many girls developing AIS [6], it is also noticeable that relaxin production rises after ovulation and declines towards menstruation. A late first menstruation delays the production of relaxin and thereby may increase collagenous cross-linking in ligaments and reduce its growth potential. Estrogen, as a last example, increases collagen turnover and decreases the stiffness of tendons and ligaments by inhibiting lysyl oxidase and decreasing cross-linking [90, 91]. A lack of estrogen, as a result of late menarche, thus enhances cross-linking and reduces growth potential of tendons and ligaments. Interestingly, levels of oestradiol are indeed reported to be lower in girls with AIS than in age-matched controls [92, 93]. Overall, a large variety of hormones interacts with tissues, functions and each other in many different ways [94]. An exploration of these from the perspective of differential growth is of great interest and a topic for future research.
Risk factors, early diagnosis and prevention
It has long been recognised that a scoliotic deformity of the spine is due to differential growth between the vertebrae and intervertebral discs on the one hand, and the interconnecting ligaments and fasciae on the other hand [18, 19, 24, 50]. If large enough, such growth discrepancy inevitably leads to twisting and bending of the spine [23, 73]. The question thus is, why ligamental growth does not keep up with spinal growth and what physical or biological cues affect this discrepancy. Such cues are essentially risk factors that may be identified and modulated to allow early diagnosis and prevention.
It appears that low muscular strength may be such a risk factor: it leads to enhanced spinal growth (Hueter–Volkmann Law), conservation of notochordal cells, increased disc pressure, and reduced bone mineral density. Also, less dynamic loading results in less microdamage in ligaments and fasciae and thereby inhibits remodelling and longitudinal growth of ligaments (Fig. 5). Girls are in general less muscular than boys, and may suffer more from severe AIS for that reason, but their susceptibility may also be related to the levels of estrogen and relaxin, which affect ligamental remodelling. There are many reasons why muscular strength may be limited: lack of exercise, reduced uptake of nutrients (eating disorder), disturbed hormone levels, disturbed motor control or proprioception, and more. While all these factors have been related to AIS, it is not the whole story: there are many girls and boys with reduced muscular strength who do not develop AIS, and there are AIS patients who seem to be physically fit. Indeed, a risk factor is not a cause or explanation, but a possible contribution to the disease. The underlying cause of AIS is multifactorial [1, 2] and the causes may differ between AIS patients. If a sufficient number of risk factors apply, the disease progresses. Differential growth provides a useful conceptual framework to think about the mechanical phenotypes of genetic, hormonal, neural, and physical cues.
Early indicators of AIS include low BMI, low muscular strength and late menarche. Of these, BMI appears to have some discriminatory power [6], and it is easy to assess by measuring length and weight. An interesting observation was made by Tomaschewsky [95], who identified an inhibited forward flexion of at least one spinal level in about 16% of 689 children 9–10 year old. This points at relatively short dorsal ligaments and could be interpreted as a first observable sign of hypo-normal flexibility and differential growth. Of the children with inhibited forward flexion, 27% developed scoliosis within one year after the assessment, where the usual incidence is about 2%. Inhibited forward flexion thus appears to be a strong early indicator of AIS that is easily assessed by a simple forward bending test. Muscular strength is relevant as argued above, but its assessment in the pre-pubertal phase has as yet not been reported. Children with low BMI, inhibited forward flexion and/or low muscular strength may be subjected to hormonal or neuromuscular screening to assess the deeper causes of AIS. Such screening should be performed well before the onset of the scoliotic deformation, because once scoliotic, it is extremely difficult to normalise the alignment of the spine.
Once an increased risk to develop AIS has been established, early treatment or even prevention should be conservative or minimally invasive. One target may be to train core stability and enhance the daily activities of the young children. Such dynamic loading will remodel the spinal ligaments, thereby avoiding locked growth. Also, it will compress the intervertebral discs, overload the notochordal cells and thereby avoid excessive osmotic pressure in the nucleus pulposus. Physical training is important for all children, but particularly important for children at risk to develop AIS. In patients with muscular dystrophy, like Duchene, physical training may be difficult or impossible. Instead, one may think of targeting the intervertebral discs, which should be considered the motor of differential growth and spinal deformity [50]. A controlled release of intradiscal pressure by degrading enzymes [96] may be considered as a minimal invasive alternative to surgical stabilization. Hormone therapies also may be considered to counteract differential growth. On the one hand, spinal growth may be modulated (e.g. by reducing growth hormone or strengthening of muscles), on the other hand ligamental remodelling may be enhanced by the suppletion of e.g. estrogen [90]. This field is largely unexplored and somewhat risky, since hormone expressions vary strongly in time and hormones tend to have multiple functions and mutual interactions.
Conclusion
AIS is a multifactorial disease, including genetic and epigenetic factors, hormones, metabolism and more [1,2,3,4]. A theory for the etio-pathogenesis of AIS, however, must be able to explain the slow, characteristic deformation of the spine, which usually occurs during the pubertal growth spurt. The conceptual framework presented here links the deformity to differential growth between the spinal column (vertebrae and intervertebral discs) and the ligaments that are attached to it. Low BMI and reduced muscular strength are identified as risk factors and may be used as early indicators of AIS, along with inhibited forward flexion. Hormones, genes, and neural disorders are factors that may underlie these risk factors and their identification should help in designing new strategies for prevention and treatment. Considering the effect of muscular strength on AIS, physical training directed at core stability may be a recommendable approach for children at risk.
References
Burwell RG, Clark EM, Dangerfield PH, Moulton A (2016) Adolescent idiopathic scoliosis (AIS): a multifactorial cascade concept for pathogenesis and embryonic origin. Scoliosis Spinal Disord 11:1–7. https://doi.org/10.1186/s13013-016-0063-1
Pérez-Machado G, Berenguer-Pascual E, Bovea-Marco M et al (2020) From genetics to epigenetics to unravel the etiology of adolescent idiopathic scoliosis. Bone 140:115563. https://doi.org/10.1016/j.bone.2020.115563
Fadzan M, Bettany-Saltikov J (2018) Etiological theories of adolescent idiopathic scoliosis: past and present. Open Orthop J 11:1466–1489. https://doi.org/10.2174/1874325001711011466
Kikanloo SR, Tarpada SP, Cho W (2019) Etiology of adolescent idiopathic scoliosis: a literature review. Asian Spine J 13:519–526. https://doi.org/10.31616/asj.2018.0096
Loncar-Dusek M, Pećina M, Prebeg Z (1991) A longitudinal study of growth velocity and development of secondary gender characteristics versus onset of idiopathic scoliosis. Clin Orthop Rel Res 270:278–282
Yim APY, Yeung HY, Hung VWY et al (2012) Abnormal skeletal growth patterns in adolescent idiopathic scoliosis—a longitudinal study until skeletal maturity. Spine (Phila Pa 1976). https://doi.org/10.1097/BRS.0b013e31825c036d
Agabegi SS, Kazemi N, Sturm PF, Mehlman CT (2015) Natural history of adolescent idiopathic scoliosis in skeletally mature patients: a critical review. J Am Acad Orthop Surg 23:714–723. https://doi.org/10.5435/JAAOS-D-14-00037
Thompson DW (1917) On growth and form. Cambridge University Press, Edinburgh
Farnum CE, Lee R, O’Hara K, Urban JPG (2002) Volume increase in growth plate chondrocytes during hypertrophy: the contribution of organic osmolytes. Bone 30:574–581. https://doi.org/10.1016/S8756-3282(01)00710-4
Villemure I, Stokes IAF (2009) Growth plate mechanics and mechanobiology: a survey of current understanding. J Biomech 42:1793–1803. https://doi.org/10.1016/j.jbiomech.2009.05.021.Growth
Ponrartana S, Fisher CL, Aggabao PC et al (2016) Small vertebral cross-sectional area and tall intervertebral disc in adolescent idiopathic scoliosis. Pediatr Radiol 46:1424–1429. https://doi.org/10.1007/s00247-016-3633-8
Brink RC, Schlösser TPC, Colo D et al (2017) Anterior spinal overgrowth is the result of the scoliotic mechanism and is located in the disc. Spine (Phila Pa 1976) 42:818–822. https://doi.org/10.1097/BRS.0000000000001919
Meir A, McNally DS, Fairbank JC et al (2008) The internal pressure and stress environment of the scoliotic intervertebral disc—a review. Proc Inst Mech Eng Part H J Eng Med 222:209–219. https://doi.org/10.1243/09544119JEIM303
Guo X, Chau WW, Chan YL, Cheng JCY (2003) Relative anterior spinal overgrowth in adolescent idiopathic scoliosis. J Bone Joint Surg Ser B 85:1026–1031. https://doi.org/10.1302/0301-620X.85B7.14046
Hueter C (1862) Anatomische studien an den extremitätengelenken neugeborener und erwachsener. Arch Pathol Anat Physiol Klin Med 25:572–599. https://doi.org/10.1007/BF01879806
Volkmann R (1862) Chirurgische erfahrungeu über knocheuverbiegungen und knochenwachsthum. Arch Pathol Anat Physiol Klin Med 24:512–540
Mehlman C, Araghi A, Roy D (1997) Hyphenated history: the Hueter–Volkmann Law. Am J Orthop 26:798–800
Somerville EW (1952) Rotational lordosis; the development of single curve. J Bone Joint Surg Br 34b3:421. https://doi.org/10.1302/0301-620x.34b3.421
Roaf R (1966) The basic anatomy of scoliosis. J Bone Joint Surg Br 48:786–792. https://doi.org/10.1302/0301-620x.48b4.786
Jarvis JG, Ashman RB, Johgnston CE, Herring JA (1988) The posterior tether in scoliosis. Clin Orthop Rel Res 227:126–134
Deacon P, Flood BM, Dickson RA (1984) Idiopathic scoliosis in three dimensions. A radiographic and morphometric analysis. J Bone Joint Surg Ser B 66:509–512. https://doi.org/10.1302/0301-620x.66b4.6746683
Dickson RA, Lawton JO, Archer IA, Butt WP (1984) The pathogenesis of idiopathic scoliosis. Biplanar spinal asymmetry. J Bone Joint Surg Ser B 66:8–15. https://doi.org/10.1302/0301-620x.66b1.6693483
Crijns TJ, Stadhouder A, Smit TH (2017) Restrained differential growth: the initiating event of adolescent idiopathic scoliosis? Spine (Phila Pa 1976) 42:E726–E732. https://doi.org/10.1097/BRS.0000000000001946
Millner PA, Dickson RA (1996) Idiopathic scoliosis: biomechanics and biology. Eur Spine J 5:362–373. https://doi.org/10.1007/BF00301963
Taylor JR (1975) Growth of human intervertebral discs and vertebral bodies. J Anat 120:49–68
Stücker R (2016) Die wachsende wirbelsäule: normale entwicklung und entwicklungsstörung. Orthopade 45:534–539. https://doi.org/10.1007/s00132-016-3277-2
De Reuver S, Costa L, Van Rheenen H et al (2022) Disc and vertebral body morphology from birth to adulthood. Spine (Phila Pa 1976) 47:E312–E318. https://doi.org/10.1097/BRS.0000000000004278
Mao SH, Jiang J, Sun X et al (2011) Timing of menarche in Chinese girls with and without adolescent idiopathic scoliosis: current results and review of the literature. Eur Spine J 20:260–265. https://doi.org/10.1007/s00586-010-1649-6
Cheung CSK, Lee WTK, Tse YK et al (2003) Abnormal peri-pubertal anthropometric measurements and growth pattern in adolescent idiopathic scoliosis: a study of 598 patients. Spine (Phila Pa 1976) 28:2152–2157. https://doi.org/10.1097/01.BRS.0000084265.15201.D5
Bala KA, Bala MM (2023) Pubertal stage-dependent anthropometric variations in Turkish children with adolescent idiopathic scoliosis: an in-depth analysis. Med Sci Monit 29:1–8. https://doi.org/10.12659/msm.940864
Chen H, Schlösser TPC, Brink RC et al (2017) The height-width-depth ratios of the intervertebral discs and vertebral bodies in adolescent idiopathic scoliosis vs controls in a Chinese population. Sci Rep 7:1–7. https://doi.org/10.1038/srep46448
Emanuel KS, Peeters M, Holewijn RM et al (2015) Poroelastic behaviour of the degenerating human intervertebral disc: a ten-day study in a loaded disc culture system. Eur Cells Mater 29:330–341
Urban JPG, McMullin JF (1985) Swelling pressure of the intervertebral disc: influence of proteoglycan and collagen contents. Biorheology 22:145–157
Emanuel KS, van der Veen AJ, Rustenburg CME et al (2018) Osmosis and viscoelasticity both contribute to time-dependent behaviour of the intervertebral disc under compressive load: a caprine in vitro study. J Biomech 70:10–15. https://doi.org/10.1016/j.jbiomech.2017.10.010
Vergroesen PPA, Van Der Veen AJ, Van Royen BJ et al (2014) Intradiscal pressure depends on recent loading and correlates with disc height and compressive stiffness. Eur Spine J 23:2359–2368. https://doi.org/10.1007/s00586-014-3450-4
Meir AR, Fairbank JCT, Jones DA et al (2007) High pressures and asymmetrical stresses in the scoliotic disc in the absence of muscle loading. Scoliosis 2:1–16. https://doi.org/10.1186/1748-7161-2-4
Tanaka A (1986) A histopathological study on the intervertebral discs of idiopathic and paralytic scoliosis—abnormalities in transition from the notochordal nucleus to the fibrocartilaginous nucleus. Nihon Seikeigeka Gakkai Zasshi 60:1227–1238
Wang Y, Bai B, Hu Y et al (2021) Hydrostatic pressure modulates intervertebral disc cell survival and extracellular matrix homeostasis via regulating Hippo-YAP/TAZ Pathway. Stem Cells Int. https://doi.org/10.1155/2021/5626487
Hong X, Zhang C, Wang F, Wu XT (2019) Large cytoplasmic vacuoles within notochordal nucleus pulposus cells: a possible regulator of intracellular pressure that shapes the cytoskeleton and controls proliferation. Cells Tissues Organs 206:9–15. https://doi.org/10.1159/000493258
Hunter CJ, Matyas JR, Duncan NA (2003) The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng 9:667–677. https://doi.org/10.1089/107632703768247368
Bach FC, Poramba-Liyanage DW, Riemers FM et al (2022) Notochordal cell-based treatment strategies and their potential in intervertebral disc regeneration. Front Cell Dev Biol 9:1–23. https://doi.org/10.3389/fcell.2021.780749
Weiler C, Nerlich AG, Schaaf R et al (2010) Immunohistochemical identification of notochordal markers in cells in the aging human lumbar intervertebral disc. Eur Spine J 19:1761–1770. https://doi.org/10.1007/s00586-010-1392-z
Li P, Gan Y, Wang H et al (2016) Dynamic compression effects on immature nucleus pulposus: a study using a novel intelligent and mechanically active bioreactor. Int J Med Sci 13:225–234. https://doi.org/10.7150/ijms.13747
Guehring T, Nerlich A, Kroeber M et al (2010) Sensitivity of notochordal disc cells to mechanical loading: an experimental animal study. Eur Spine J 19:113–121. https://doi.org/10.1007/s00586-009-1217-0
Wang F, Gao ZX, Cai F et al (2017) Formation, function, and exhaustion of notochordal cytoplasmic vacuoles within intervertebral disc: current understanding and speculation. Oncotarget 8:57800–57812. https://doi.org/10.18632/oncotarget.18101
Galileo G (1914) Two new sciences. The MacMillan Company, New York
Smit TH (2002) The use of a quadruped as an in vivo model for the study of the spine—biomechanical considerations. Eur Spine J 11:137–144. https://doi.org/10.1007/s005860100346
Busscher I, Van Dieën JH, Kingma I et al (2009) Biomechanical characteristics of different regions of the human spine: an in vitro study on multilevel spinal segments. Spine (Phila Pa 1976) 34:2858–2864. https://doi.org/10.1097/BRS.0b013e3181b4c75d
Smit TH, Van Tunen MS, Van Der Veen AJ et al (2011) Quantifying intervertebral disc mechanics: a new definition of the neutral zone. BMC Musculoskelet Disord 12:38. https://doi.org/10.1186/1471-2474-12-38
Smit TH (2020) Adolescent idiopathic scoliosis: the mechanobiology of differential growth. JOR Spine 3:1–13. https://doi.org/10.1002/jsp2.1115
Dickson RA (1988) The aetiology of spinal deformities. Lancet 331:1151–1155. https://doi.org/10.1016/S0140-6736(88)91963-0
Schoenau E, Neu CM, Beck B et al (2002) Bone mineral content per muscle cross-sectional area as an index of the functional muscle-bone unit. J Bone Miner Res 17:1095–1101. https://doi.org/10.1359/jbmr.2002.17.6.1095
Cook SD, Harding AF, Morgan EL et al (1987) Trabecular bone mineral density in idiopathic scoliosis. J Pediatr Orthop 7(2):168
Lee WTK, Cheung CSK, Tse YK et al (2005) Association of osteopenia with curve severity in adolescent idiopathic scoliosis: a study of 919 girls. Osteoporos Int 16:1924–1932. https://doi.org/10.1007/s00198-005-1964-7
Ramos O, Razzouk J, Chung J et al (2022) Opportunistic assessment of bone density in patients with adolescent idiopathic scoliosis using MRI-based vertebral bone quality. J Clin Neurosci 103:41–43
Barrios C, Cortés S, Pérez-Encinas C et al (2011) Anthropometry and body composition profile of girls with nonsurgically treated adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 36:1470–1477. https://doi.org/10.1097/BRS.0b013e3181f55083
Bulik CM, Reba L, Siega-Riz AM, Reichborn-Kjennerud T (2005) Anorexia nervosa: definition, epidemiology, and cycle of risk. Int J Eat Disord. https://doi.org/10.1002/eat.20107
Alborghetti A, Scimeca G, Costanzo G, Boca S (2008) The prevalence of eating disorders in adolescents with idiopathic scoliosis. Eat Disord 16:85–93. https://doi.org/10.1080/10640260701773660
Miyagi M, Saito W, Imura T et al (2021) Body composition in Japanese girls with adolescent idiopathic scoliosis. Spine Surg Relat Res 5:68–74. https://doi.org/10.22603/SSRR.2020-0088
Starčević-Klasan G, Cvijanović O, Peharec S et al (2008) Anthropometric parameters as predictors for iliopsoas muscle strength in healthy girls and in girls with adolescent idiopathic scoliosis. Coll Antropol 32:461–466
Shahidi B, Yoo A, Farnsworth C et al (2021) Paraspinal muscle morphology and composition in adolescent idiopathic scoliosis: a histological analysis. JOR Spine 4:1–7. https://doi.org/10.1002/jsp2.1169
Eisner LE, Rosario R, Andarawis-Puri N, Arruda EM (2022) The role of the non-collagenous extracellular matrix in tendon and ligament mechanical behavior: a review. J Biomech Eng. https://doi.org/10.1115/14053086
Thorpe CT, Birch HL, Clegg PD, Screen HRC (2013) The role of the non-collagenous matrix in tendon function. Int J Exp Pathol 94:248–259. https://doi.org/10.1111/iep.12027
Birk DE, Nurminskaya MV, Zycband EI (1995) Collagen fibrillogenesis in situ: fibril segments undergo post-depositional modifications resulting in linear and lateral growth during matrix development. Dev Dyn 202:229–243. https://doi.org/10.1002/aja.1002020303
Vesentini S, Redaelli A, Montevecchi FM (2005) Estimation of the binding force of the collagen molecule-decorin core protein complex in collagen fibril. J Biomech 38:433–443. https://doi.org/10.1016/j.jbiomech.2004.04.032
Wood ML, Lester GE, Dahners LE (1998) Collagen fiber sliding during ligament growth and contracture. J Orthop Res 16:438–440. https://doi.org/10.1002/jor.1100160407
Stauber T, Blache U, Snedeker JG (2020) Tendon tissue microdamage and the limits of intrinsic repair. Matrix Biol 85–86:68–79. https://doi.org/10.1016/j.matbio.2019.07.008
Dahners L, Sykes K, Muller P (2013) A study of the mechanisms influencing ligament growth. Orthopedics 12:1569–1572
Huang C, Yannas IV (1977) Mechanochemical studies of enzymatic degradation of insoluble collagen fibers. J Biomed Mater Res 11:137–154. https://doi.org/10.1002/jbm.820110113
Ghazanfari S, Driessen-Mol A, Bouten CVC, Baaijens FPT (2016) Modulation of collagen fiber orientation by strain-controlled enzymatic degradation. Acta Biomater 35:118–126. https://doi.org/10.1016/j.actbio.2016.02.033
Saini K, Cho S, Dooling LJ, Discher DE (2020) Tension in fibrils suppresses their enzymatic degradation—a molecular mechanism for ‘use it or lose it.’ Matrix Biol 85–86:34–46. https://doi.org/10.1016/j.matbio.2019.06.001
Erisken C, Tsiantis A, Papathanasiou TD, Karvelas EG (2020) Collagen fibril diameter distribution affects permeability of ligament tissue: a computational study on healthy and injured tissues. Comput Methods Programs Biomed 196:105554. https://doi.org/10.1016/j.cmpb.2020.105554
Lovett RW (1903) A contribution to the study of mechanics of the spine. Am J Anat 4:457–462
Erwin WM, Ashman K, O’Donnel P, Inman RD (2006) Nucleus pulposus notochord cells secrete connective tissue growth factor and up-regulate proteoglycan expression by intervertebral disc chondrocytes. Arthritis Rheumatol 54:3859–3867. https://doi.org/10.1002/art.22258
Vergroesen PPA, Emanuel KS, Peeters M et al (2018) Are axial intervertebral disc biomechanics determined by osmosis? J Biomech 70:4–9. https://doi.org/10.1016/j.jbiomech.2017.04.027
Wise CA, Sepich D, Ushiki A et al (2020) The cartilage matrisome in adolescent idiopathic scoliosis. Bone Res. https://doi.org/10.1038/s41413-020-0089-0
Mccallum-loudeac JAD (2021) Insights into spinal cord biology and its contributions to adolescent idiopathic scoliosis: from GWAS to animal models. University of Otago, Dunedin
Machida M, Saito M, Dubousset J et al (2005) Pathological mechanism of idiopathic scoliosis: experimental scoliosis in pinealectomized rats. Eur Spine J 14:843–848. https://doi.org/10.1007/s00586-004-0806-1
Wood ML, Luthin WN, Lester GE, Dahners LE (2003) Tendon creep is potentiated by NKISK and relaxin which produce collagen fiber sliding. Iowa Orthop J 23:75–79
Skogland LB, Miller JAA (1980) Growth related hormones in idiopathic scoliosis: an endocrine basis for accelerated growth. Acta Orthop 51:779–789. https://doi.org/10.3109/17453678008990874
Ahl T, Albertsson-Wikland K, Kalém R (1988) Twenty-four-hour growth hormone profiles in pubertal girls with idiopathic scoliosis. Spine (Phila Pa 1976) 13:139–142
Benyi E, Sävendahl L (2017) The physiology of childhood growth: hormonal regulation. Horm Res Paediatr 88:6–14. https://doi.org/10.1159/000471876
Batin S, Ekinci Y, Gürbüz K et al (2022) The role of pineal gland volume in the development of scoliosis. Eur Spine J 32:181–189
Latalski M, Danielewicz-Bromberek A, Fatyga M et al (2017) Current insights into the aetiology of adolescent idiopathic scoliosis. Arch Orthop Trauma Surg 137:1327–1333. https://doi.org/10.1007/s00402-017-2756-1
Girardo M, Bettini N, Dema E, Cervellati S (2011) The role of melatonin in the pathogenesis of adolescent idiopathic scoliosis (AIS). Eur Spine J 20:68–74. https://doi.org/10.1007/s00586-011-1750-5
Gargano G, Oliva F, Migliorini C, Maffulli N (2022) Melatonin and adolescent idiopathic scoliosis: the present evidence. Surg 20:e315–e321
Ng KY, Leong MK, Liang H, Paxinos G (2017) Melatonin receptors: distribution in mammalian brain and their respective putative functions. Brain Struct Funct 222:2921–2939. https://doi.org/10.1007/s00429-017-1439-6
Manto M, Bower JM, Conforto AB et al (2012) Consensus paper: roles of the cerebellum in motor control-the diversity of ideas on cerebellar involvement in movement. Cerebellum 11:457–487. https://doi.org/10.1007/s12311-011-0331-9
Dehghan F, Haerian BS, Muniandy S et al (2014) The effect of relaxin on the musculoskeletal system. Scand J Med Sci Sports 24:220–229. https://doi.org/10.1111/sms.12149
Hansen M, Kongsgaard M, Holm L et al (2009) Effect of estrogen on tendon collagen synthesis, tendon structural characteristics, and biomechanical properties in postmenopausal women. J Appl Physiol 106:1385–1393. https://doi.org/10.1152/japplphysiol.90935.2008
Chidi-Ogbolu N, Baar K (2019) Effect of estrogen on musculoskeletal performance and injury risk. Front Physiol. https://doi.org/10.3389/fphys.2018.01834
Kulis A, Goździalska A, Drąg J et al (2015) Participation of sex hormones in multifactorial pathogenesis of adolescent idiopathic scoliosis. Int Orthop 39:1227–1236. https://doi.org/10.1007/s00264-015-2742-6
Esposito T, Uccello R, Caliendo R et al (2009) Estrogen receptor polymorphism, estrogen content and idiopathic scoliosis in human: a possible genetic linkage. J Steroid Biochem Mol Biol 116:56–60. https://doi.org/10.1016/j.jsbmb.2009.04.010
Liang ZT, Guo CF, Li J, Zhang HQ (2021) The role of endocrine hormones in the pathogenesis of adolescent idiopathic scoliosis. FASEB J 35:1–14. https://doi.org/10.1096/fj.202100759R
Tomaschewski R (1989) Die idiopathische skoliose in der sagittalebene. Beitr Orthop Traumatol 36:520–529. https://doi.org/10.1055/s-2008-1039818
Hoogendoorn RJ, Wuisman PI, Smit TH et al (2007) Experimental intervertebral disc degeneration induced by chondroitinase ABC in the goat. Spine (Phila Pa 1976) 32:1816–1825. https://doi.org/10.1097/BRS.0b013e31811ebac5
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Smit, T.H. On growth and scoliosis. Eur Spine J (2024). https://doi.org/10.1007/s00586-024-08276-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00586-024-08276-9