Abstract
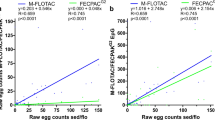
This study focuses on the comparison of three selected modifications of the McMaster counting technique, namely the McMaster method modified by Wetzel (W) and Zajíček (Z), as well as the concentration McMaster technique according to Roepstorff and Nansen (R&N). These modifications differ in the weights of faeces examined (W, 2 g/Z, 1 g/R&N, 4 g), flotation solutions (W, NaCl/Z, MgSO4 + Na2S2O3/R&N, NaCl + glucose), centrifugation (W, none/Z, 2,000 RPM for 2 min and 2,000 RPM for 1 min/R&N, 1,200 RPM for 5 min), number of McMaster chambers investigated (W, 3/Z, 2/R&N, 2), and multiplication factors used (W, 67/Z, 33/R&N, 20). To investigate the sensitivity and reliability of these methods, nematode eggs (Teladorsagia circumcincta) were used. Parasite elements are distributed through negative binomial distribution in naturally infected host faeces, and the number of parasite elements in a given amount of faeces sample is unknown to man. Therefore, we decided to prepare the exact number of eggs which were added to the parasite negative faeces; the faecal sample was then investigated. From this perspective, this is the first time a comparison of the McMaster methods has been so accurately investigated. This approach allows us to evaluate the real sensitivity and reliability of the tested method. As the findings of this study indicate, the highest sensitivity and reliability were obtained using the Roepstorff and Nansen modification. This McMaster modification is able to detect 20 eggs per sample (in 70% of samples). Concentrations of 200 and 500 eggs can be found in almost 100% of samples. Moreover, this method is simple, cheap and fast. For these reasons, we can recommend this method for routine veterinary practice.


Similar content being viewed by others
References
Agneessens J, Claerebout E, Vercruysse J (2001) Development of a copro-antigen capture ELISA for detecting Ostertagia ostertagi infections in cattle. Vet Parasitol 97:227–238
Bondarenko IG, Kinčeková J, Várady M, Königová A, Kuchta M, Koňáková G (2009) Use of modified McMaster method for the diagnosis of intestinal helminth infections and estimating parasitic egg load in human faecal samples in non-endemic areas. Helminthologia 46:62–64
Bott NJ, Campbell BE, Beveridge I, Chilton NB, Rees D, Hunt PW, Gasser RB (2009) A combined microscopic-molecular method for the diagnosis of strongylid infections in sheep. Int J Parasitol 39:1277–1287
Bowman DD, Lynn RC (1999) Georgi’s parasitology for veterinarians, 7th edn. W.B. Saunders, Philadelphia
Breza M (1959) The improvement of the swine faeces coproovoscopic examination methodology using a new flotation solution and mucagel (article in Czech). Vet Čas 8:569–576
Broussard JD (2003) Optimal fecal assessment. Clin Tech Small Anim Pract 18:218–230
Chiodini PL (2005) New diagnostics in parasitology. Infect Dis Clin N Am 19:267–270
Coles GC, Bauer C, Borgsteede FHM, Geerts S, Klei TR, Taylor MA, Waller PJ (1992) World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol 44:35–44
Cringoli G, Rinaldi L, Veneziano V, Capelli G, Scala A (2004) The influence of flotation solution, sample dilution and the choice of McMaster slide area (volume) on the reliability of the McMaster technique in estimating the faecal egg counts of gastrointestinal strongyles and Dicrocoelium dendriticum in sheep. Vet Parasitol 123:121–131
Cringoli G, Rinaldi L, Maurelli MP, Utzinger J (2010) FLOTAC: new multivalent techniques for quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat Protoc 5:503–515
Deplazes P, Gottstein B, Eckert J, Jenkins DJ, Ewald D, Jimenez-Palacios S (1992) Detection of Echinococcus coproantigens by enzyme-linked immunosorbent assay in dogs, dingoes and foxes. Parasitol Res 78:303–308
Dunn A, Keymer A (1986) Factors affecting the reliability of the McMaster technique. J Helminthol 60:260–262
Eysker M, Ploeger HW (2000) Value of present diagnostic methods for gastrointestinal nematode infections in ruminants. Parasitology 120:109–119
Flohr C, Tuyen LN, Lewis S, Minh TT, Campbell J, Britton J, Williams H, Hien TT, Farrar J, Quinnell RJ (2007) Low efficacy of mebendazole against hookworm in Vietnam: two randomized controlled trials. Am J Trop Med Hyg 76:732–736
Foreyt WJ (2001) Veterinary parasitology: reference manual, 5th edn. Wiley-Blackwell, Iowa
Gasbarre LC, Leighton EA, Bryant D (1996) Reliability of a single fecal egg per gram determination as a measure of individual and herd values for trichostrongyle nematodes of cattle. Am J Vet Res 57:168–171
Gates M, Nolan TJ (2009) Comparison of passive fecal flotation run by veterinary students to zinc-sulfate centrifugation flotation run in a diagnostic parasitology laboratory. J Parasitol 95:1213–1214
Harmon AF, Williams ZB, Zarlenga DS, Hildreth MB (2007) Real-time PCR for quantifying Haemonchus contortus eggs and potential limiting factors. Parasitol Res 101:71–76
Humbert JF, Cabaret J (1995) Use of random amplified polymorphic DNA for identification of ruminant trichostrongylid nematodes. Parasitol Res 81:1–5
Johnson MJ, Behnke JM, Coles GC (1996) Detection of gastrointestinal nematodes by a coproantigen capture ELISA. Res Vet Sci 60:7–12
Karamon J, Ziomko I, Cencek T, Sroka J (2008) Modified flotation method with the use of Percoll for the detection of Isospora suis oocysts in suckling piglet faeces. Vet Parasitol 156:324–328
Learmount J, Conyers C, Hird H, Morgan C, Craig BH, von Samson-Himmelstjerna G, Taylor M (2009) Development and validation of real-time PCR methods for diagnosis of Teladorsagia circumcincta and Haemonchus contortus in sheep. Vet Parasitol 166:268–274
MAFF (1986) Manual of Veterinary Parasitological Laboratory Techniques, Fisheries and Food Reference Book, Vol. 418. Ministry of Agriculture, HMSO, London
Mes THM (2003) Technical variability and required sample size of helminth egg isolation procedures. Vet Parasitol 115:311–320
Mes THM, Ploeger HW, Terlou M, Kooyman FNJ, van der Ploeg MPJ, Eysker M (2001) A novel method for the isolation of gastro-intestinal nematode eggs that allows automated analysis of digital images of egg preparation and high throughput screening. Parasitology 123:309–314
Morgan ER, Cavill L, Curry GE, Wood RM, Mitchell ESE (2005) Effects of aggregation and sample size on composite faecal egg counts in sheep. Vet Parasitol 131:79–87
Nichols J, Obendorf DL (1994) Application of a composite faecal egg count procedure in diagnostic parasitology. Vet Parasitol 52:337–342
Pereckiene A, Kaziunaite V, Vyšniauskas A, Petkevicius S, Malakauskas A, Šarkunas M, Taylor MA (2007) A comparison of modifications of the McMaster method for the enumeration of Ascaris suum eggs in pig faecal samples. Vet Parasitol 149:111–116
Rinaldi L, Russo T, Schioppi M, Pennacchio S, Cringoli G (2007) Passalurus ambiguus: new insights into copromicroscopic diagnosis and circadian rhythm of egg excretion. Parasitol Res 101:557–561
Roeber F, Jex AR, Campbell AJ, Campbell BE, Anderson GA, Gasser RB (2011) Evaluation and application of a molecular method to assess the composition of strongylid nematode populations in sheep with naturally acquired infections. Infect Genet Evol. doi:10.1016/j.meegid.2011.01.013
Roepstorff A (1998) Natural Ascaris suum infections in swine diagnosed by coprological and serological (ELISA) methods. Parasitol Res 84:537–543
Roepstorff A, Nansen P (1998) Epidemiology, diagnosis and control of helminth parasites of swine. FAO Animal Health Manual, Rome
Schnieder T, Heise M, Epe C (1999) Genus-specic PCR for the differentiation of eggs or larvae from gastrointestinal nematodes of ruminants. Parasitol Res 85:895–898
StatSoft, Inc (2009) STATISTICA (data analysis software system), version 9.0. www.statsoft.com
Stephenson LS, Latham MC, Kurz KM, Kinoti SN, Brigham H (1989) Treatment with a single dose of albendazole improves growth of Kenyan schoolchildren with hookworm, Trichuris trichiura, and Ascaris lumbricoides infections. Am J Trop Med Hyg 41:78–87
Ward MP, Lyndal-Murphy M, Baldock FC (1997) Evaluation of a composite method for counting helminth eggs in cattle faeces. Vet Parasitol 73:181–187
Wetzel E (1951) Verbesserte McMaster-Kammer zum Auszählen von Wurmeiern. Tierärztl Umsch 6:209–210
Wood IB, Amaral NK, Bairden K, Duncan JL, Kassai T, Malone JB, Pankavich JA, Reinecke RK, Slocombe O, Taylor SM, Vercruysse J (1995) World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) second edition of guidelines for evaluating the efficacy of anthelmintics in ruminants (bovine, ovine, caprine). Vet Parasitol 58:181–213
Zajíček D (1978) Comparision of the efficiency of two quantitative ovoskopic methods (article in Czech). Vet Med 23:275–280
Zarlenga DS, Barry Chute M, Gasbarre LC, Boyd PC (2001) A multiplex PCR assay for differentiating economically important gastrointestinal nematodes of cattle. Vet Parasitol 97:199–209
Acknowledgement
The authors wish to acknowledge Brian Kavalír for his assistance in proofreading the manuscript.
Ethical standards
All experiments conducted with laboratory animals comply with the current laws of the country in which they were performed.
Conflicts of interest
This study was supported by the Research Project of the Faculty of Agrobiology, Food and Natural Resources, Czech University of Life Sciences Prague, No. MSM 6046070901.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vadlejch, J., Petrtýl, M., Zaichenko, I. et al. Which McMaster egg counting technique is the most reliable?. Parasitol Res 109, 1387–1394 (2011). https://doi.org/10.1007/s00436-011-2385-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2385-5