Abstract
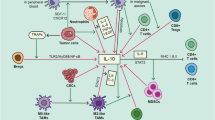
The approval of the first specific drug catumaxomab for the treatment of malignant ascites is the subject of this review. This trifunctional antibody is known to kill EpCAM-positive tumor cells and therefore attacks the primary cause of malignant ascites formation in the peritoneal cavity. Until today catumaxomab is the only EpCam-targeted antibody approved by the European Medicines Agency. Ovarian cancer is caused by epithelial tumors cells which overexpress epithelial cell adhesion molecule (EpCAM). The existing literature concerning the use of catumaxomab for the treatment of malignant ascites associated with ovarian cancer until today is reported in this article. It is very encouraging that different prospective studies from diverse scientific teams recently presented positive results concerning the efficacy and the safety of catumaxomab in the treatment of malignant ascites in patients with ovarian cancer. A case of a patient with ovarian cancer FIGO IIIc is also referred in this article. A complete remission and stable disease was found after 4 i.p. infusions of catumaxomab.
Similar content being viewed by others
References
Bookman MA (2009) Trials with impact on clinical management: first line. Int J Gynecol Cancer 19(Suppl 2):S55–S62 (review)
Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R, Gynecologic Oncology Group (2003) Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol 21(17):3194–3200
Monk BJ, Choi DC, Pugmire G, Burger RA (2005) Activity of bevacizumab (rhuMAB VEGF) in advanced refractory epithelial ovarian cancer. Gynecol Oncol 96(3):902–905
Cannistra SA (2010) Evaluating new regimens in recurrent ovarian cancer: how much evidence is good enough? J Clin Oncol 28(19):3101–3103
Cannistra SA (2002) Is there a “best” choice of second-line agent in the treatment of recurrent, potentially platinum-sensitive ovarian cancer? J Clin Oncol 20(5):1158–1160
Malayev Y, Levene R, Gonzalez F (2012) Palliative chemotherapy for malignant ascites secondary to ovarian cancer. Am J Hosp Palliat Care 29(7):515–521
Barni S, Cabiddu M, Ghilardi M, Petrelli F (2011) A novel perspective for an orphan problem: old and new drugs for the medical management of malignant ascites. Crit Rev Oncol Hematol 79(2):144–153. doi:10.1016/j.critrevonc.2010.07.016
Shen-Gunther J, Mannel RS (2002) Ascites as a predictor of ovarian malignancy. Gynecol Oncol 87(1):77–83
Ayantunde AA, Parsons SL (2007) Pattern and prognostic factors in patients with malignant ascites: a retrospective study. Ann Oncol 18(5):945–949
Woopen H, Sehouli J (2009) Current and future options in the treatment of malignant ascites in ovarian cancer. Anticancer Res 29(8):3353–3359
Smith EM, Jayson GC (2003) The current and future management of malignant ascites. Clin Oncol (R Coll Radiol) 15(2):59–72 (review)
Xu L, Yoneda J, Herrera C, Wood J, Killion JJ, Fidler IJ (2000) Inhibition of malignant ascites and growth of human ovarian carcinoma by oral administration of a potent inhibitor of the vascular endothelial growth factor receptor tyrosine kinases. Int J Oncol 16(3):445–454
Beattie GJ, Smyth JF (1998) Phase I study of intraperitoneal metalloproteinase inhibitor BB94 in patients with malignant ascites. Clin Cancer Res 4(8):1899–1902
Kaufmann M, Schmid H, Raeth U, Grischke EM, Kempeni J, Schlick E, Bastert G (1990) Therapy of ascites with tumor necrosis factor in ovarian cancer. Geburtshilfe Frauenheilkd 50(9):678–682
Freedman RS, Kudelka AP, Kavanagh JJ, Verschraegen C, Edwards CL, Nash M, Levy L, Atkinson EN, Zhang HZ, Meelichar B, Patenia R, Templin S, Scott W, Platsoucas CD (2000) Clinical and biological effects of intraperitoneal injections of recombinant interferon-gamma and recombinant interleukin 2 with or without tumor-specific infiltrating lymphocytes in patients with ovarian or peritoneal carcinoma. Clin Cancer Res 6(6):2268–2278
Walker J, Armstrong DK, Huang HQ, Fowler J, Webster K, Burger RA, Clarke Pearson D (2006) Intraperitoneal catheter outcomes in a phase 3 trial of intravenous versus intraperitoneal chemotherapy in an optimal stage 3 ovarian and primary peritoneal cancer: a Gynaecologic Oncology group study. Gynecol Oncol 100:27–32
Friedman RS, Deavers M, Liu J et al (2004) Peritoneal inflammation—a microenvironment for epithelial ovarian cancer (EOC). J Transl Med 2:23
Revill Mely N, Bozzo J (2008) Catumaxomab anti-Ep-CAM monoclonal antibody. Drugs Future 33(5):385–391
Linke R, Klein A, Seimetz D (2010) Catumaxomab: clinical development and future directions. MAbs 2(2):129–136
Zeidler R, Mysliwietz J, Csanady M et al (2000) The Fc-region of a new class of intact biospecific antibody mediates activation of accessory cells and NK Cells and induces phagocytosis tumor cells. Br J Cancer 83:261–266
Riesenberg R, Buchner A, Pohla H et al (2001) Lysis of prostate carcinoma cells by trifunctional bispecific antibodies (AEp-CAM X aCD3). J Histochem Cytochem 49:911–917
Schmitt M, Schmitt A, Reinhardt P, Thess B, Manfra B, Lindhofer H, Riechelmann H, Wiesneth M, Gronau S (2004) Opsonisation with a trifunctional bispecific (aCD3 × aEpCAM) antibody results in efficient lysis in vitro and in vivo of EpCAM-positive tumor cells by cytotoxic T lymphocytes. Int J Oncol 25(4):841–848
Balzar M, Winter MJ, de Boer CJ, Litvinov SV (1999) The biology of the 17–1A antigen (Ep-CAM). J Mol Med (Berl). 77(10):699–712 (review)
Reichert JM, Valge-Archer VE (2007) Development trends for monoclonal antibody cancer therapeutics. Nat Rev Drug Discov 6(5):349–356 (review)
Heiss MM, Stroehlein MA, Jager M et al (2005) Immunotherapy of malignant ascites with trifunctional antibodies. Int J Cancer 117(3):435–443
Burges A, Wimberger P, Kümper C, Gorbounova V, Sommer H, Schmalfeldt B, Pfisterer J, Lichinitser M, Makhson A, Moiseyenko V, Lahr A, Schulze E, Jäger M, Ströhlein MA, Heiss MM, Gottwald T, Lindhofer H, Kimmig R (2007) Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM × anti-CD3 antibody: a phase I/II study. Clin Cancer Res 13(13):3899–3905
Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, Dudnichenko AS, Aleknaviciene B, Razbadauskas A, Gore M, Ganea-Motan E, Ciuleanu T, Wimberger P, Schmittel A, Schmalfeldt B, Burges A, Bokemeyer C, Lindhofer H, Lahr A, Parsons SL (2010) The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer 127(9):2–2209
Wimberger P, Gilet H, Gonschior A-K, Heiss MM, Moehler M, Oskay-Oezcelik G, Al-Batran S-E, Schmalfeldt B, Schmittel A, Schulze E, Parsons SL (2012) Deterioration in quality of life (QoL) in patients with malignant ascites: results from a phase II/III study comparing paracentesis plus catumaxomab with paracentesis alone. Ann Oncol 23(8):1979–1985
Removab® Summary of product characteristics, Sept. 2011. http://www.ema.europa.eu/
Pietzner K, Chekerov R, Reinthaller A, Reimer D, Reimer T, Angleitner-Boubenizek L, Tschirschmann M, Lindhofer H, Braicu EI, Fotopoulou C, Sehouli J (2012) Matched pair analysis of intra- and postoperative catumaxomab in patients with ovarian cancer from a multicenter, single-arm phase II trial versus a consecutive single-center collective of ovarian cancer patients without immunotherapy. J Clin Oncol 30(Suppl; abstr 5080)
Papanikolaou G, Fotopoulou C, Braicu I, Chekerov R, Schmidt SC, Pietzner K, Sehouli J (2011) First surgical experience of intraperitoneal treatment with the trifunctional antibody catumaxomab (anti-EpCam × anti-CD3) for epithelial ovarian cancer. Anticancer Res 31(8):2603–2608
Woopen H, Pietzner K, Darb-Esfahani S, Oskay-Oezcelik G, Sehouli J (2012) Extraperitoneal response to intraperitoneal immunotherapy with catumaxomab in a patient with cutaneous lymphangiosis carcinomatosa from ovarian cancer: a case report and review of the literature. Med Oncol 29(5):3416–3420
Petrelli F, Borgonovo K, Lonati V, Elia S, Barni S (2012) Regression of liver metastases after treatment with intraperitoneal catumaxomab for malignant ascites due to breast cancer. Targt Oncol [Epub ahead of print]
Pietzner K, Jäger M, Schoberth A, Oskay-Özcelik G, Kuhberg M, Lindhofer H, Sehouli J (2012) First patient treated with a re-challenge of catumaxomab in recurrent malignant ascites: a case report. Med Oncol 29(2):1391–1396
Chekerov R, Reinthaller A, Reimer D, Reimer T, Angleitner-Boubenizek L, Halfen M, Lindhofer H, Braicu I, Oskay-özcelik G,Sehouli J (2010) Intraoperative immunotherapy with the trifunctional antibody catumaxomab in patients with ovarian cancer: results from a phase II study. American Society of Clinical Oncology, vol 28, no 15_suppl (May 20 Supplement), p 5039
Ruf P, Kluge M, Jäger M, Burges A, Volovat C, Heiss MM, Hess J, Wimberger P, Brandt B, Lindhofer H (2010) Pharmacokinetics, immunogenicity and bioactivity of the therapeutic antibody catumaxomab intraperitoneally administered to cancer patients. Br J Clin Pharmacol 69(6):617–625
Ströhlein MA, Siegel R, Jäger M, Lindhofer H, Jauch KW, Heiss MM (2009) Induction of anti-tumor immunity by trifunctional antibodies in patients with peritoneal carcinomatosis. J Exp Clin Cancer Res 14(28):18
Belau A, Pfisterer J, Wimpberger P, Kurzeder C, Du Bois A, Schouli J, Loibl S, Burchardi N (2007) Randomized, multicenter, two dose level open label phase II a study with the intraperitoneally infused trifunctional bispecific antibody catumaxomab to select the better dose level in platinum refractory epithelial ovarian cancer patients. J Clin Oncol 25(185) (June 20 Supplement 5556) (Part 1)
Stroehlein MA, Lordick F, Ruettinger D, Gruetzner U, Menzel H, Bartelheim K, Jaeger M, Lindhofer H (2005) Treatment of peritoneal carcinomatosis due to GI tract cancer by intraperitoneal application of the trifunctional antibody catumaxomab: results of a phase I/II trial. J Clin Oncol 2005 ASCP Annual Meeting Proceedings, 23(165) (June 1 Supplement 2529) (Part I of II)
Conflict of interest
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsikouras, P., Tsagias, N., Pinidis, P. et al. The contribution of catumaxomab in the treatment of malignant ascites in patients with ovarian cancer: a review of the literature. Arch Gynecol Obstet 288, 581–585 (2013). https://doi.org/10.1007/s00404-013-2868-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-013-2868-y