Abstract
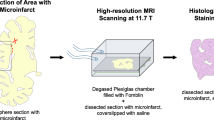
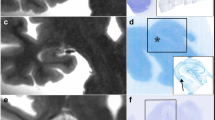
Small subclinical hyperintense lesions are frequently encountered on brain diffusion-weighted imaging (DWI) scans of patients with cerebral amyloid angiopathy (CAA). Interpretation of these DWI+ lesions, however, has been limited by absence of histopathological examination. We aimed to determine whether DWI+ lesions represent acute microinfarcts on histopathology in brains with advanced CAA, using a combined in vivo MRI—ex vivo MRI—histopathology approach. We first investigated the histopathology of a punctate cortical DWI+ lesion observed on clinical in vivo MRI 7 days prior to death in a CAA case. Subsequently, we assessed the use of ex vivo DWI to identify similar punctate cortical lesions post-mortem. Intact formalin-fixed hemispheres of 12 consecutive cases with CAA and three non-CAA controls were subjected to high-resolution 3 T ex vivo DWI and T2 imaging. Small cortical lesions were classified as either DWI+/T2+ or DWI−/T2+. A representative subset of lesions from three CAA cases was selected for detailed histopathological examination. The DWI+ lesion observed on in vivo MRI could be matched to an area with evidence of recent ischemia on histopathology. Ex vivo MRI of the intact hemispheres revealed a total of 130 DWI+/T2+ lesions in 10/12 CAA cases, but none in controls (p = 0.022). DWI+/T2+ lesions examined histopathologically proved to be acute microinfarcts (classification accuracy 100%), characterized by presence of eosinophilic neurons on hematoxylin and eosin and absence of reactive astrocytes on glial fibrillary acidic protein-stained sections. In conclusion, we suggest that small DWI+ lesions in CAA represent acute microinfarcts. Furthermore, our findings support the use of ex vivo DWI as a method to detect acute microinfarcts post-mortem, which may benefit future histopathological investigations on the etiology of microinfarcts.





Similar content being viewed by others
References
Arvanitakis Z, Capuano AW, Leurgans SE, Buchman AS, Bennett DA, Schneider JA (2017) The relationship of cerebral vessel pathology to brain microinfarcts. Brain Pathol 27:77–85. https://doi.org/10.1111/bpa.12365
Auriel E, Gurol ME, Ayres A, Dumas AP, Schwab KM, Vashkevich A et al (2012) Characteristic distributions of intracerebral hemorrhage-associated diffusion-weighted lesions. Neurology 79:2335–2341. https://doi.org/10.1212/WNL.0b013e318278b66f
Batool S, O’Donnell M, Sharma M, Islam S, Dagenais GR, Poirier P et al (2014) Incidental magnetic resonance diffusion-weighted imaging-positive lesions are rare in neurologically asymptomatic community-dwelling adults. Stroke 45:2115–2117. https://doi.org/10.1161/strokeaha.114.005782
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 9:179–194
Duering M, Adam R, Wollenweber FA, Bayer-Karpinska A, Baykara E, Cubillos-Pinilla LY et al (2019) Within-lesion heterogeneity of subcortical DWI lesion evolution, and stroke outcome: a voxel-based analysis. J Cereb Blood Flow Metab. https://doi.org/10.1177/0271678x19865916
Dyrby TB, Baare WF, Alexander DC, Jelsing J, Garde E, Sogaard LV (2011) An ex vivo imaging pipeline for producing high-quality and high-resolution diffusion-weighted imaging datasets. Hum Brain Mapp 32:544–563. https://doi.org/10.1002/hbm.21043
Ferro DA, van den Brink H, Exalto LG, Boomsma JM, Barkhof F, Prins ND et al (2019) Clinical relevance of acute cerebral microinfarcts in vascular cognitive impairment. Neurology 92:e1558–e1566
Garcia JH, Yoshida Y, Chen H, Li Y, Zhang ZG, Lian J et al (1993) Progression from ischemic injury to infarct following middle cerebral artery occlusion in the rat. Am J Pathol 142:623–635
Greenberg SM, Charidimou A (2018) Diagnosis of cerebral amyloid angiopathy: evolution of the Boston criteria. Stroke 49:491–497. https://doi.org/10.1161/strokeaha.117.016990
Gregoire SM, Charidimou A, Gadapa N, Dolan E, Antoun N, Peeters A et al (2011) Acute ischaemic brain lesions in intracerebral haemorrhage: multicentre cross-sectional magnetic resonance imaging study. Brain 134:2376–2386. https://doi.org/10.1093/brain/awr172
Havsteen I, Ovesen C, Willer L, Nybing JD, Ægidius K, Marstrand J et al (2018) Small cortical grey matter lesions show no persistent infarction in transient ischaemic attack? A prospective cohort study. BMJ Open 8:e018160
Hilal S, Baaij LGA, de Groot M, Niessen WJ, Ikram MK, Ikram MA et al (2019) Prevalence and clinical relevance of diffusion-weighted imaging lesions: the Rotterdam study. Neurology 93:e1058–e1067. https://doi.org/10.1212/wnl.0000000000008090
Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC et al (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s Dement 8:1–13. https://doi.org/10.1016/j.jalz.2011.10.007
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841
Jeon JH, Jung HW, Jang HM, Moon JH, Park KT, Lee HC et al (2015) Canine model of ischemic stroke with permanent middle cerebral artery occlusion: clinical features, magnetic resonance imaging, histopathology, and immunohistochemistry. J Vet Sci 16:75–85
Karperien A, Ahammer H, Jelinek HF (2013) Quantitating the subtleties of microglial morphology with fractal analysis. Front Cell Neurosci 7:3. https://doi.org/10.3389/fncel.2013.00003
Kelly PJ, Hedley-Whyte ET, Primavera J, He J, Gonzalez RG (2001) Diffusion MRI in ischemic stroke compared to pathologically verified infarction. Neurology 56:914–920. https://doi.org/10.1212/wnl.56.7.914
Kimberly WT, Gilson A, Rost NS, Rosand J, Viswanathan A, Smith EE et al (2009) Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology 72:1230–1235. https://doi.org/10.1212/01.wnl.0000345666.83318.03
Leemans A, Jeurissen B, Sijbers J, Jones D (2009) ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. In: Proceedings of the international society for magnetic resonance in medicine, p 3537
Love S, Chalmers K, Ince P, Esiri M, Attems J, Jellinger K et al (2014) Development, appraisal, validation and implementation of a consensus protocol for the assessment of cerebral amyloid angiopathy in post-mortem brain tissue. Am J Neurodegener Dis 3:19–32
McNab JA, Jbabdi S, Deoni SC, Douaud G, Behrens TE, Miller KL (2009) High resolution diffusion-weighted imaging in fixed human brain using diffusion-weighted steady state free precession. Neuroimage 46:775–785. https://doi.org/10.1016/j.neuroimage.2009.01.008
Mena H, Cadavid D, Rushing EJ (2004) Human cerebral infarct: a proposed histopathologic classification based on 137 cases. Acta Neuropathol 108:524–530. https://doi.org/10.1007/s00401-004-0918-z
Menon RS, Burgess RE, Wing JJ, Gibbons MC, Shara NM, Fernandez S et al (2012) Predictors of highly prevalent brain ischemia in intracerebral hemorrhage. Ann Neurol 71:199–205. https://doi.org/10.1002/ana.22668
Miller KL, Stagg CJ, Douaud G, Jbabdi S, Smith SM, Behrens TEJ et al (2011) Diffusion imaging of whole, post-mortem human brains on a clinical MRI scanner. Neuroimage 57:167–181. https://doi.org/10.1016/j.neuroimage.2011.03.070
Minematsu K, Li L, Fisher M, Sotak CH, Davis MA, Fiandaca M (1992) Diffusion-weighted magnetic resonance imaging: rapid and quantitative detection of focal brain ischemia. Neurology 42:235
Okamoto Y, Ihara M, Fujita Y, Ito H, Takahashi R, Tomimoto H (2009) Cortical microinfarcts in Alzheimer’s disease and subcortical vascular dementia. NeuroReport 20:990–996
RESTART Collaboration (2019) Effects of antiplatelet therapy after stroke due to intracerebral haemorrhage (RESTART): a randomised, open-label trial. Lancet 393:2613–2623. https://doi.org/10.1016/s0140-6736(19)30840-2
Schulz UG, Flossmann E, Francis JM, Redgrave JN, Rothwell PM (2007) Evolution of the diffusion-weighted signal and the apparent diffusion coefficient in the late phase after minor stroke: a follow-up study. J Neurol 254:375–383. https://doi.org/10.1007/s00415-006-0381-y
Shih AY, Hyacinth HI, Hartmann DA, van Veluw SJ (2018) Rodent models of cerebral microinfarct and microhemorrhage. Stroke 49:803–810. https://doi.org/10.1161/strokeaha.117.016995
Smith EE, Schneider JA, Wardlaw JM, Greenberg SM (2012) Cerebral microinfarcts: the invisible lesions. Lancet Neurol 11:272–282. https://doi.org/10.1016/s1474-4422(11)70307-6
Soontornniyomkij V, Lynch MD, Mermash S, Pomakian J, Badkoobehi H, Clare R et al (2010) Cerebral microinfarcts associated with severe cerebral beta-amyloid angiopathy. Brain Pathol 20:459–467. https://doi.org/10.1111/j.1750-3639.2009.00322.x
Summers PM, Hartmann DA, Hui ES, Nie X, Deardorff RL, McKinnon ET et al (2017) Functional deficits induced by cortical microinfarcts. J Cereb Blood Flow Metab. https://doi.org/10.1177/0271678x16685573
ter Telgte A, Wiegertjes K, Gesierich B, Marques JP, Huebner M, de Klerk JJ et al (2019) Contribution of acute infarcts to cerebral small vessel disease progression. Ann Neurol 86:582–592. https://doi.org/10.1002/ana.25556
van Veluw SJ, Zwanenburg JJ, Engelen-Lee J, Spliet WG, Hendrikse J, Luijten PR et al (2013) In vivo detection of cerebral cortical microinfarcts with high-resolution 7T MRI. J Cereb Blood Flow Metab 33:322–329. https://doi.org/10.1038/jcbfm.2012.196
van Veluw SJ, Charidimou A, van der Kouwe AJ, Lauer A, Reijmer YD, Costantino I et al (2016) Microbleed and microinfarct detection in amyloid angiopathy: a high-resolution MRI-histopathology study. Brain 139:3151–3162. https://doi.org/10.1093/brain/aww229
van Veluw SJ, Lauer A, Charidimou A, Bounemia N, Xiong L, Boulouis G et al (2017) Evolution of DWI lesions in cerebral amyloid angiopathy: evidence for ischemia. Neurology 89:2136–2142. https://doi.org/10.1212/wnl.0000000000004668
van Veluw SJ, Shih AY, Smith EE, Chen C, Schneider JA, Wardlaw JM et al (2017) Detection, risk factors, and functional consequences of cerebral microinfarcts. Lancet Neurol 16:730–740. https://doi.org/10.1016/s1474-4422(17)30196-5
van Veluw SJ, Reijmer YD, van der Kouwe AJ, Charidimou A, Riley GA, Leemans A et al (2019) Histopathology of diffusion imaging abnormalities in cerebral amyloid angiopathy. Neurology 92:e933–e943. https://doi.org/10.1212/wnl.0000000000007005
van Veluw SJ, Scherlek AA, Freeze WM, ter Telgte A, van der Kouwe AJ, Bacskai BJ et al (2019) Different microvascular alterations underlie microbleeds and microinfarcts. Ann Neurol 86:279–292. https://doi.org/10.1002/ana.25512
Viswanathan A, Greenberg SM (2011) Cerebral amyloid angiopathy in the elderly. Ann Neurol 70:871–880. https://doi.org/10.1002/ana.22516
von Kummer R, Dzialowski I (2017) Imaging of cerebral ischemic edema and neuronal death. Neuroradiology 59:545–553. https://doi.org/10.1007/s00234-017-1847-6
Wang M, Iliff JJ, Liao Y, Chen MJ, Shinseki MS, Venkataraman A et al (2012) Cognitive deficits and delayed neuronal loss in a mouse model of multiple microinfarcts. J Neurosci 32:17948–17960. https://doi.org/10.1523/jneurosci.1860-12.2012
Wiegertjes K, ter Telgte A, Oliveira PB, van Leijsen EMC, Bergkamp MI, van Uden IWM et al (2019) The role of small diffusion-weighted imaging lesions in cerebral small vessel disease. Neurology. https://doi.org/10.1212/wnl.0000000000008364
Acknowledgements
The authors would like to thank the families of the patients who generously donated their brains to our research studies. The authors would also like to thank Dr. Ana Amaral and Dr. Patrick Dooley for their excellent technical assistance and Dr. Alberto Serrano-Pozo for helpful discussions. The work described in this study was supported by the Van Leersum Grant of the Royal Netherlands Academy of Arts and Sciences and an Alzheimer Nederland fellowship to AtT, and the National Institutes of Health (NINDS R01 NS096730, NIA K99 AG059893, NINDS RF1 NS110054, and NIA R21 AG046657). During this study, SJvV received funding from the Netherlands Organisation for Scientific Research (Veni Grant 91619021). MD received funding from the Radboud Excellence Initiative (18U.018651). FEdL was supported by a clinical established investigator grant of the Dutch Heart Foundation (Grant 2014 T060), and by a VIDI innovational grant from The Netherlands Organisation for Health Research and Development, ZonMw (Grant 016126351).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
ter Telgte, A., Scherlek, A.A., Reijmer, Y.D. et al. Histopathology of diffusion-weighted imaging-positive lesions in cerebral amyloid angiopathy. Acta Neuropathol 139, 799–812 (2020). https://doi.org/10.1007/s00401-020-02140-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-020-02140-y