Abstract
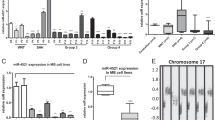
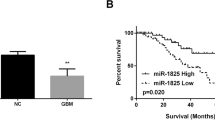
Overexpression of high mobility group AT-hook 1 (HMGA1) is common in human cancers. Little is known about the mechanisms underlying its deregulation and downstream targets, and information about its clinical and biological significance in medulloblastoma (MB) is lacking. Here, we demonstrated frequent genomic gain at 6p21.33–6p21.31 with copy number increase leading to overexpression of HMGA1 in MB. The overexpression correlated with a high proliferation index and poor prognosis. Moreover, we found that hsa-miR-124a targeted 3′UTR of HMGA1 and negatively modulated the expression in MB cells, indicating that loss/downregulation of hsa-miR-124a reported in our previous study could contribute to the overexpression. Regarding the biological significance of HMGA1, siRNA knockdown and ectopic expression studies revealed the crucial roles of HMGA1 in controlling MB cell growth and migration/invasion through modulation of apoptosis and formation of filopodia and stress fibers, respectively. Furthermore, we identified cdc25A as a target of HMGA1 and showed that physical interaction between HMGA1 and the cdc25A promoter is required for transcriptional upregulation. In clinical samples, HMGA1 and cdc25A were concordantly overexpressed. Functionally, cdc25A is involved in the HMGA1-mediated control of MB cell growth. Finally, netropsin, which competes with HMGA1 in DNA binding, reduced the expression of cdc25A by suppression of its promoter activity and inhibited in vitro and in vivo intracranial MB cell growth. In conclusion, our results delineate the mechanisms underlying the deregulation and reveal the functional significance of HMGA1 in controlling MB cell growth and migration/invasion. Importantly, the results highlight the therapeutic potential of targeting HMGA1 in MB patients.








Similar content being viewed by others
References
Abe N, Watanabe T, Masaki T et al (2000) Pancreatic duct cell carcinomas express high levels of high mobility group I(Y) proteins. Cancer Res 60:3117–3122
Abe N, Watanabe T, Sugiyama M et al (1999) Determination of high mobility group I(Y) expression level in colorectal neoplasias: a potential diagnostic marker. Cancer Res 59:1169–1174
Adesina AM, Nguyen Y, Mehta V et al (2007) FOXG1 dysregulation is a frequent event in medulloblastoma. J Neurooncol 85:111–122
Akai T, Ueda Y, Sasagawa Y et al (2004) High mobility group I-C protein in astrocytoma and glioblastoma. Pathol Res Pract 200:619–624
Balcerczak M, Pasz-Walczak G, Balcerczak E, Wojtylak M, Kordek R, Mirowski M (2003) HMGI(Y) gene expression in colorectal cancer: comparison with some histological typing, grading, and clinical staging. Pathol Res Pract 199:641–646
Birchmeier W, Birchmeier C (1995) Epithelial–mesenchymal transitions in development and tumor progression. EXS 74:1–15
Chang ZG, Yang LY, Wang W et al (2005) Determination of high mobility group A1 (HMGA1) expression in hepatocellular carcinoma: a potential prognostic marker. Dig Dis Sci 50:1764–1770
Chiappetta G, Avantaggiato V, Visconti R et al (1996) High level expression of the HMGI (Y) gene during embryonic development. Oncogene 13:2439–2446
Chiappetta G, Manfioletti G, Pentimalli F et al (2001) High mobility group HMGI(Y) protein expression in human colorectal hyperplastic and neoplastic diseases. Int J Cancer 91:147–151
Chiappetta G, Tallini G, De Biasio MC et al (1998) Detection of high mobility group I HMGI(Y) protein in the diagnosis of thyroid tumors: HMGI(Y) expression represents a potential diagnostic indicator of carcinoma. Cancer Res 58:4193–4198
Cleynen I, Huysmans C, Sasazuki T, Shirasawa S, Van de Ven W, Peeters K (2007) Transcriptional control of the human high mobility group A1 gene: basal and oncogenic Ras-regulated expression. Cancer Res 67:4620–4629
Cozzi P (2001) Distamycin derivatives as potential anticancer agents. IDrugs 4:573–581
De Bortoli M, Castellino RC, Lu XY et al (2006) Medulloblastoma outcome is adversely associated with overexpression of EEF1D, RPL30, and RPS20 on the long arm of chromosome 8. BMC Cancer 6:223
Donato G, Martinez Hoyos J, Amorosi A et al (2004) High mobility group A1 expression correlates with the histological grade of human glial tumors. Oncol Rep 11:1209–1213
Eberhart CG, Kratz J, Wang Y et al (2004) Histopathological and molecular prognostic markers in medulloblastoma: c-myc, N-myc, TrkC, and anaplasia. J Neuropathol Exp Neurol 63:441–449
Esposito F, Pierantoni GM, Battista S et al (2009) Interaction between HMGA1 and retinoblastoma protein is required for adipocyte differentiation. J Biol Chem 284:25993–26004
Fedele M, Bandiera A, Chiappetta G et al (1996) Human colorectal carcinomas express high levels of high mobility group HMGI(Y) proteins. Cancer Res 56:1896–1901
Ferretti E, De Smaele E, Po A et al (2009) MicroRNA profiling in human medulloblastoma. Int J Cancer 124:568–577
Giangaspero F, Bigner SH, Kleihues P, Pietsch T, Trojanowski JQ (2000) Medulloblastoma. In: Kleihues P, Cavenee WK (eds) World Health Organization classification of tumours. Pathology and genetics-tumors of the nervous system. LARC, Lyon, pp 129–137
Grant MA, Baron RM, Macias AA, Layne MD, Perrella MA, Rigby AC (2009) Netropsin improves survival from endotoxaemia by disrupting HMGA1 binding to the NOS2 promoter. Biochem J 418:103–112
Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP (2007) MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 27:91–105
Hernan R, Fasheh R, Calabrese C et al (2003) ERBB2 up-regulates S100A4 and several other prometastatic genes in medulloblastoma. Cancer Res 63:140–148
Hillion J, Wood LJ, Mukherjee M et al (2009) Upregulation of MMP-2 by HMGA1 promotes transformation in undifferentiated, large-cell lung cancer. Mol Cancer Res 7:1803–1812
Hristov AC, Cope L, Di Cello F et al (2010) HMGA1 correlates with advanced tumor grade and decreased survival in pancreatic ductal adenocarcinoma. Mod Pathol 23:98–104
Huth JR, Bewley CA, Nissen MS et al (1997) The solution structure of an HMG-I(Y)-DNA complex defines a new architectural minor groove binding motif. Nat Struct Biol 4:657–665
Kaddar T, Rouault JP, Chien WW et al (2009) Two new miR-16 targets: caprin-1 and HMGA1, proteins implicated in cell proliferation. Biol Cell 101:511–524
Ke N, Albers A, Claassen G et al (2004) One-week 96-well soft agar growth assay for cancer target validation. Biotechniques 36:826–828, 830, 832–833
Kim DH, Park YS, Park CJ et al (1999) Expression of the HMGI(Y) gene in human colorectal cancer. Int J Cancer 84:376–380
Li KK, Pang JC, Ching AK et al (2009) miR-124 is frequently down-regulated in medulloblastoma and is a negative regulator of SLC16A1. Hum Pathol 40:1234–1243
Liau SS, Rocha F, Matros E, Redston M, Whang E (2008) High mobility group AT-hook 1 (HMGA1) is an independent prognostic factor and novel therapeutic target in pancreatic adenocarcinoma. Cancer 113:302–314
Lo KC, Rossi MR, Eberhart CG, Cowell JK (2007) Genome wide copy number abnormalities in pediatric medulloblastomas as assessed by array comparative genome hybridization. Brain Pathol 17:282–296
McCabe MG, Ichimura K, Liu L et al (2006) High-resolution array-based comparative genomic hybridization of medulloblastomas and supratentorial primitive neuroectodermal tumors. J Neuropathol ExpNeurol 65:549–561
Nam ES, Kim DH, Cho SJ et al (2003) Expression of HMGI(Y) associated with malignant phenotype of human gastric tissue. Histopathology 42:466–471
Northcott PA, Nakahara Y, Wu X et al (2009) Multiple recurrent genetic events converge on control of histone lysine methylation in medulloblastoma. Nat Genet 41:465–472
Pierson J, Hostager B, Fan R, Vibhakar R (2008) Regulation of cyclin dependent kinase 6 by microRNA 124 in medulloblastoma. J Neurooncol 90:1–7
Pomeroy SL, Tamayo P, Gaasenbeek M et al (2002) Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature 415:436–442
Ray D, Kiyokawa H (2008) CDC25A phosphatase: a rate-limiting oncogene that determines genomic stability. Cancer Res 68:1251–1253
Ray D, Terao Y, Nimbalkar D et al (2007) Hemizygous disruption of Cdc25A inhibits cellular transformation and mammary tumorigenesis in mice. Cancer Res 67:6605–6611
Reeves R (2010) Nuclear functions of the HMG proteins. Biochim Biophys Acta 1799:3–14
Reeves R, Edberg DD, Li Y (2001) Architectural transcription factor HMGI(Y) promotes tumor progression and mesenchymal transition of human epithelial cells. Mol Cell Biol 21:575–594
Rho YS, Lim YC, Park IS et al (2007) High mobility group HMGI(Y) protein expression in head and neck squamous cell carcinoma. Acta Otolaryngol 127:76–81
Sandelin A, Wasserman WW, Lenhard B (2004) ConSite: web-based prediction of regulatory elements using cross-species comparison. Nucleic Acids Res 32(Web Server issue):W249–W252
Sarhadi VK, Wikman H, Salmenkivi K et al (2006) Increased expression of high mobility group A proteins in lung cancer. J Pathol 209:206–212
Shih JY, Lee YC, Yang SC, Hong TM, Huang CY, Yang PC (2003) Collapsin response mediator protein-1: a novel invasion-suppressor gene. Clin Exp Metastasis 20:69–76
Stearns D, Chaudhry A, Abel TW, Burger PC, Dang CV, Eberhart CG (2006) c-Myc overexpression causes anaplasia in medulloblastoma. Cancer Res 66:673–681
Tong CY, Hui AB, Yin XL et al (2004) Detection of oncogene amplifications in medulloblastomas by comparative genomic hybridization and array-based comparative genomic hybridization. J Neurosurg 100:187–193
van der Zee JA, ten Hagen TL, Hop WC et al (2010) Differential expression and prognostic value of HMGA1 in pancreatic head and periampullary cancer. Eur J Cancer 46:3393–3399
Wegner M, Grummt F (1990) Netropsin, distamycin and berenil interact differentially with a high-affinity binding site for the high mobility group protein HMG-I. Biochem Biophys Res Commun 166:1110–1117
Wei JJ, Wu X, Peng Y et al (2011) Regulation of HMGA1 expression by microRNA-296 affects prostate cancer growth and invasion. Clin Cancer Res 17:1297–1305
Wood LJ, Maher JF, Bunton TE, Resar LM (2000) The oncogenic properties of the HMG-I gene family. Cancer Res 60:4256–4261
Wood LJ, Mukherjee M, Dolde CE et al (2000) HMG-I/Y, a new c-Myc target gene and potential oncogene. Mol Cell Biol 20:5490–5502
Acknowledgments
This work was supported by Hong Kong Research Grant Council General Research Funds 469211 to KML and 472307 to HKN.
Author information
Authors and Affiliations
Corresponding author
Additional information
K.-M. Lau and Q. K. Y. Chan equally contributed to the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lau, KM., Chan, Q.K.Y., Pang, J.C.S. et al. Overexpression of HMGA1 deregulates tumor growth via cdc25A and alters migration/invasion through a cdc25A-independent pathway in medulloblastoma. Acta Neuropathol 123, 553–571 (2012). https://doi.org/10.1007/s00401-011-0934-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-011-0934-8