Abstract
Purpose
Pomegranate and walnuts are widely consumed dietary sources and contain several bioactive compounds, including the ellagitannins (ETs). ETs are polyphenols that are metabolized in the gut microbiota to urolithin A (UA). p53 is a tumor suppressor that lost its activity through MDM2 activation in about half cancers. The purpose of this study was to investigate the influence of UA on the p53-MDM2 interaction pathway in prostate cancer cell lines.
Methods
Three human prostate cancer cell lines were used that harbor different p53 genotypes; LNCaP (p53+/+), 22RV1(p53−/+) and PC3 (p53−/−). Cell viability was determined by CellTiter-Glo Luminescent assay. Apoptosis was confirmed by measuring annexin V by flow cytometry. The expression of p53, its target proteins, and apoptotic markers were measured by western blotting. Real-time qPCR was used to measure the gene expression of p21, a main target gene of p53. Co-immunoprecipitation–immunoblotting was used to assess the inhibition of interactions between p53 and MDM2 and to assess the effect of UA on MDM2-mediated p53 polyubiquitination.
Results
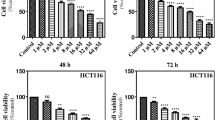
We found UA inhibited CaP cells’ viability and induced apoptosis. For 22RV1 and LNCaP, we found UA increased p53 protein expression and its main target protein, p21, and MDM2, forming an autoregulatory feedback loop. In addition, UA increased the p53 proapoptotic proteins PUMA and NOXA. Moreover, UA inhibited the interaction between p53 and MDM2 and inhibited MDM2-mediated p53 polyubiquitination. UA downregulated MDM2 and XIAP protein expression in PC3 cells and upregulated p21 and p14ARF in a p53-independent manner.
Conclusion
The influencing of UA on p53-MDM2 pathway may partly contribute to its anticancer effect.








Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386. https://doi.org/10.1002/ijc.29210
Culig Z, Santer FR (2014) Androgen receptor signaling in prostate cancer. Cancer Metastasis Rev 33(2):413–427. https://doi.org/10.1007/s10555-013-9474-0
Logan IR, McNeill HV, Cook S, Lu X, Lunec J, Robson CN (2007) Analysis of the MDM2 antagonist nutlin-3 in human prostate cancer cells. Prostate 67(8):900–906. https://doi.org/10.1002/pros.20568
Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF (2008) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol 26(2):242–245. https://doi.org/10.1200/jco.2007.12.4008
Chappell WH, Lehmann BD, Terrian DM, Abrams SL, Steelman LS, McCubrey JA (2012) p53 expression controls prostate cancer sensitivity to chemotherapy and the MDM2 inhibitor nutlin-3. Cell Cycle 11(24):4579–4588. https://doi.org/10.4161/cc.22852
Gupta K, Thakur VS, Bhaskaran N, Nawab A, Babcook MA, Jackson MW, Gupta S (2012) Green tea polyphenols induce p53-dependent and p53-independent apoptosis in prostate cancer cells through two distinct mechanisms. PLoS One 7(12):e52572. https://doi.org/10.1371/journal.pone.0052572
Kastenhuber ER, Lowe SW (2017) Putting p53 in context. Cell 170(6):1062–1078. https://doi.org/10.1016/j.cell.2017.08.028
Khoo KH, Verma CS, Lane DP (2014) Drugging the p53 pathway: understanding the route to clinical efficacy. Nat Rev Drug Discov 13(3):217–236. https://doi.org/10.1038/nrd4236
Nakano K, Vousden KH (2001) PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 7(3):683–694
Dai C, Gu W (2010) p53 post-translational modification: deregulated in tumorigenesis. Trends Mol Med 16(11):528–536. https://doi.org/10.1016/j.molmed.2010.09.002
Kruse JP, Gu W (2008) SnapShot: p53 posttranslational modifications. Cell 133(5):930–930.e931. https://doi.org/10.1016/j.cell.2008.05.020
Kruse JP, Gu W (2009) Modes of p53 regulation. Cell 137(4):609–622. https://doi.org/10.1016/j.cell.2009.04.050
Munoz-Fontela C, Gonzalez D, Marcos-Villar L, Campagna M, Gallego P, Gonzalez-Santamaria J, Herranz D, Gu W, Serrano M, Aaronson SA, Rivas C (2011) Acetylation is indispensable for p53 antiviral activity. Cell Cycle 10(21):3701–3705. https://doi.org/10.4161/cc.10.21.17899
Sakaguchi K, Herrera JE, Saito S, Miki T, Bustin M, Vassilev A, Anderson CW, Appella E (1998) DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev 12(18):2831–2841
Jimenez GS, Khan SH, Stommel JM, Wahl GM (1999) p53 regulation by post-translational modification and nuclear retention in response to diverse stresses. Oncogene 18(53):7656–7665. https://doi.org/10.1038/sj.onc.1203013
Lee JT, Gu W (2010) The multiple levels of regulation by p53 ubiquitination. Cell Death Differ 17(1):86–92. https://doi.org/10.1038/cdd.2009.77
Yuan J, Luo K, Zhang L, Cheville JC, Lou Z (2010) USP10 regulates p53 localization and stability by deubiquitinating p53. Cell 140(3):384–396. https://doi.org/10.1016/j.cell.2009.12.032
Nag S, Qin J, Srivenugopal KS, Wang M, Zhang R (2013) The MDM2-p53 pathway revisited. J Biomed Res 27(4):254–271. https://doi.org/10.7555/jbr.27.20130030
Bohlman S, Manfredi JJ (2014) p53-independent effects of Mdm2. Sub-cell Biochem 85:235–246. https://doi.org/10.1007/978-94-017-9211-0_13
Khan N, Bharali DJ, Adhami VM, Siddiqui IA, Cui H, Shabana SM, Mousa SA, Mukhtar H (2014) Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis 35(2):415–423. https://doi.org/10.1093/carcin/bgt321
Robson CH, Ganapathy M, Swanson GP, Natarajan M, Papanikolaou N, Hanes MA, Yeh IT, Ghosh R, Kumar AP (2009) Phellodendron amurense bark extract enhances radiosensitivity by inhibition of nf-kappa B in transgenic adenocarcinoma of mouse prostate model and human prostate cancer cells. J Urol 181(4):479. https://doi.org/10.1016/S0022-5347(09)61356-2
Caderni G, De Filippo C, Luceri C, Salvadori M, Giannini A, Biggeri A, Remy S, Cheynier V, Dolara P (2000) Effects of black tea, green tea and wine extracts on intestinal carcinogenesis induced by azoxymethane in F344 rats. Carcinogenesis 21(11):1965–1969. https://doi.org/10.1093/carcin/21.11.1965
Corona G, Deiana M, Incani A, Vauzour D, Dessi MA, Spencer JP (2009) Hydroxytyrosol inhibits the proliferation of human colon adenocarcinoma cells through inhibition of ERK1/2 and cyclin D1. Mol Nutr Food Res 53(7):897–903. https://doi.org/10.1002/mnfr.200800269
Mantena SK, Baliga MS, Katiyar SK (2006) Grape seed proanthocyanidins induce apoptosis and inhibit metastasis of highly metastatic breast carcinoma cells. Carcinogenesis 27(8):1682–1691. https://doi.org/10.1093/carcin/bgl030
Granci V, Dupertuis YM, Pichard C (2010) Angiogenesis as a potential target of pharmaconutrients in cancer therapy. Curr Opin Clin Nutr Metab Care 13(4):417–422. https://doi.org/10.1097/MCO.0b013e3283392656
Paller CJ, Pantuck A, Carducci MA (2017) A review of pomegranate in prostate cancer. Prostate Cancer Prostatic Dis 20(3):265–270. https://doi.org/10.1038/pcan.2017.19
Reiter RJ, Tan DX, Manchester LC, Korkmaz A, Fuentes-Broto L, Hardman WE, Rosales-Corral SA, Qi W (2013) A walnut-enriched diet reduces the growth of LNCaP human prostate cancer xenografts in nude mice. Cancer Investig 31(6):365–373. https://doi.org/10.3109/07357907.2013.800095
Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, Heber D (2005) In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem 16(6):360–367. https://doi.org/10.1016/j.jnutbio.2005.01.006
Landete JM (2011) Ellagitannins, ellagic acid and their derived metabolites: a review about source, metabolism, functions and health. Food Res Int 44(5):1150–1160. https://doi.org/10.1016/j.foodres.2011.04.027
Espin JC, Larrosa M, Garcia-Conesa MT, Tomas-Barberan F (2013) Biological significance of urolithins, the gut microbial ellagic Acid-derived metabolites: the evidence so far. Evid Based Complement Altern Med 2013:270418. https://doi.org/10.1155/2013/270418
Sanchez-Gonzalez C, Ciudad CJ, Noe V, Izquierdo-Pulido M (2014) Walnut polyphenol metabolites, urolithins A and B, inhibit the expression of the prostate-specific antigen and the androgen receptor in prostate cancer cells. Food Funct 5(11):2922–2930. https://doi.org/10.1039/c4fo00542b
Vicinanza R, Zhang Y, Henning SM, Heber D (2013) Pomegranate juice metabolites, ellagic acid and urolithin A, synergistically inhibit androgen-independent prostate cancer cell growth via distinct effects on cell cycle control and apoptosis. Evid Based Complement Altern Med 2013:247504. https://doi.org/10.1155/2013/247504
Soliman E, Van Dross R (2016) Anandamide-induced endoplasmic reticulum stress and apoptosis are mediated by oxidative stress in non-melanoma skin cancer: receptor-independent endocannabinoid signaling. Mol Carcinog 55(11):1807–1821. https://doi.org/10.1002/mc.22429
Meek DW, Knippschild U (2003) Posttranslational modification of MDM2. Mol Cancer Res 1(14):1017–1026
Agrawal A, Yang J, Murphy RF, Agrawal DK (2006) Regulation of the p14ARF-Mdm2-p53 pathway: an overview in breast cancer. Exp Mol Pathol 81(2):115–122. https://doi.org/10.1016/j.yexmp.2006.07.001
Sanchez-Gonzalez C, Ciudad CJ, Izquierdo-Pulido M, Noe V (2016) Urolithin A causes p21 up-regulation in prostate cancer cells. Eur J Nutr 55(3):1099–1112. https://doi.org/10.1007/s00394-015-0924-z
Loughery J, Cox M, Smith LM, Meek DW (2014) Critical role for p53-serine 15 phosphorylation in stimulating transactivation at p53-responsive promoters. Nucleic Acids Res 42(12):7666–7680. https://doi.org/10.1093/nar/gku501
Valentine JM, Kumar S, Moumen A (2011) A p53-independent role for the MDM2 antagonist Nutlin-3 in DNA damage response initiation. BMC Cancer 11:79. https://doi.org/10.1186/1471-2407-11-79
Pise-Masison CA, Radonovich M, Sakaguchi K, Appella E, Brady JN (1998) Phosphorylation of p53: a novel pathway for p53 inactivation in human T-cell lymphotropic virus type 1-transformed cells. J Virol 72(8):6348–6355
Sramkoski RM, Pretlow TG 2nd, Giaconia JM, Pretlow TP, Schwartz S, Sy MS, Marengo SR, Rhim JS, Zhang D, Jacobberger JW (1999) A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell Dev Biol Anim 35(7):403–409. https://doi.org/10.1007/s11626-999-0115-4
Lehmann BD, McCubrey JA, Jefferson HS, Paine MS, Chappell WH, Terrian DM (2007) A dominant role for p53-dependent cellular senescence in radiosensitization of human prostate cancer cells. Cell Cycle 6(5):595–605. https://doi.org/10.4161/cc.6.5.3901
Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC (2005) Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 17(3):393–403. https://doi.org/10.1016/j.molcel.2004.12.030
Sparks A, Dayal S, Das J, Robertson P, Menendez S, Saville MK (2014) The degradation of p53 and its major E3 ligase Mdm2 is differentially dependent on the proteasomal ubiquitin receptor S5a. Oncogene 33(38):4685–4696. https://doi.org/10.1038/onc.2013.413
Shukla S, Gupta S (2008) Apigenin-induced prostate cancer cell death is initiated by reactive oxygen species and p53 activation. Free Radic Biol Med 44(10):1833–1845. https://doi.org/10.1016/j.freeradbiomed.2008.02.007
Aliouat-Denis CM, Dendouga N, Van den Wyngaert I, Goehlmann H, Steller U, van de Weyer I, Van Slycken N, Andries L, Kass S, Luyten W, Janicot M, Vialard JE (2005) p53-independent regulation of p21Waf1/Cip1 expression and senescence by Chk2. Mol Cancer Res 3(11):627–634. https://doi.org/10.1158/1541-7786.mcr-05-0121
Zhang Z, Li M, Wang H, Agrawal S, Zhang R (2003) Antisense therapy targeting MDM2 oncogene in prostate cancer: effects on proliferation, apoptosis, multiple gene expression, and chemotherapy. Proc Natl Acad Sci USA 100(20):11636–11641. https://doi.org/10.1073/pnas.1934692100
Wang H, Yu D, Agrawal S, Zhang R (2003) Experimental therapy of human prostate cancer by inhibiting MDM2 expression with novel mixed-backbone antisense oligonucleotides: in vitro and in vivo activities and mechanisms. Prostate 54(3):194–205. https://doi.org/10.1002/pros.10187
Zhang Z, Wang H, Li M, Agrawal S, Chen X, Zhang R (2004) MDM2 is a negative regulator of p21WAF1/CIP1, independent of p53. J Biol Chem 279(16):16000–16006. https://doi.org/10.1074/jbc.M312264200
Gu L, Zhu N, Zhang H, Durden DL, Feng Y, Zhou M (2009) Regulation of XIAP translation and induction by MDM2 following irradiation. Cancer Cell 15(5):363–375. https://doi.org/10.1016/j.ccr.2009.03.002
Wu RC, Schonthal AH (1997) Activation of p53–p21waf1 pathway in response to disruption of cell-matrix interactions. J Biol Chem 272(46):29091–29098
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest related to this manuscript.
Rights and permissions
About this article
Cite this article
Mohammed Saleem, Y.I., Albassam, H. & Selim, M. Urolithin A induces prostate cancer cell death in p53-dependent and in p53-independent manner. Eur J Nutr 59, 1607–1618 (2020). https://doi.org/10.1007/s00394-019-02016-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-019-02016-2