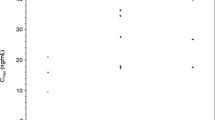Abstract
Purpose
This study was conducted to define the maximum tolerated dose (MTD), recommended phase two dose (RPTD), and toxicities of gemcitabine + dasatinib (GD) and gemcitabine + dasatinib + cetuximab (GDC) in advanced solid tumor patients.
Methods
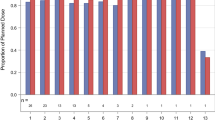
This study was a standard phase I 3 + 3 dose escalation study evaluating two combination regimens, GD and GDC. Patients with advanced solid tumors were enrolled in cohorts of 3–6 to either GD or GDC. Gemcitabine was dosed at 1000 mg/m2 weekly for 3 of 4 weeks, dasatinib was dosed in mg PO BID, and cetuximab was dosed at 250 mg/m2 weekly after a loading dose of cetuximab of 400 mg/m2. There were two dose levels for dasatinib: (1) gemcitabine + dasatinib 50 mg ± cetuximab, and (2) gemcitabine + dasatinib 70 mg ± cetuximab. Cycle length was 28 days. Standard cycle 1 dose-limiting toxicity (DLT) definitions were used. Eligible patients had advanced solid tumors, adequate organ and marrow function, and no co-morbidities that would increase the risk of toxicity. Serum, plasma, and skin biopsy biomarkers were obtained pre- and on-treatment.
Results
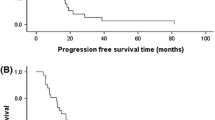
Twenty-five patients were enrolled, including 21 with pancreatic adenocarcinoma. Three patients received prior gemcitabine. Twenty-one patients were evaluable for toxicity and 16 for response. Four DLTs were observed: Grade (Gr) 3 neutropenia (GDC1, n = 1), Gr 3 ALT (GD2, n = 2), and Gr 5 pneumonitis (GDC2, n = 1). Possible treatment-emergent adverse events (TEAEs) in later cycles included: Gr 3–4 neutropenia (n = 7), Gr 4 colitis (n = 1), Gr 3 bilirubin (n = 2), Gr 3 anemia (n = 2), Gr 3 thrombocytopenia (n = 2), Gr 3 edema/fluid retention (n = 1), and Gr 3 vomiting (n = 3). Six of 16 patients (3 of whom were gemcitabine-refractory) had stable disease (SD) as best response, median duration = 5 months (range 1–7). One gemcitabine-refractory patient had a partial response (PR). Median PFS was 2.9 months (95% CI 2.1, 5.8). Median OS was 5.8 months (95% CI 4.1, 11.8). Dermal wound biopsies demonstrated that dasatinib resulted in a decrease of total and phospho-Src levels, and cetuximab resulted in a decrease of EGFR and ERBB2 levels.
Conclusions
The MTD/RPTD of GD is gemcitabine 1000 mg/m2 weekly for 3 of 4 weeks and dasatinib 50 mg PO BID. The clinical activity of GD seen in this study was modest, and does not support its further investigation in pancreatic cancer.



Similar content being viewed by others
References
Siegel RL et al (2018) Cancer statistics, 2018. CA Cancer J Clin 68(1):7–30
Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) (2018) SEER Cancer Statistics Review, 1975–2015, National Cancer Institute, Bethesda, MD. https://seer.cancer.gov/csr/1975_2015/
Plunkett W et al (1995) Gemcitabine: metabolism, mechanisms of action, and self-potentiation. Semin Oncol 22(4 Suppl 11):3–10
Burris HA III et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15(6):2403–2413
Flossmann-Kast BB et al (1998) Src stimulates insulin-like growth factor I (IGF-I)-dependent cell proliferation by increasing IGF-I receptor number in human pancreatic carcinoma cells. Cancer Res 58(16):3551–3554
Tanno S et al (2001) AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res 61(2):589–593
Menke A et al (2001) Down-regulation of E-cadherin gene expression by collagen type I and type III in pancreatic cancer cell lines. Cancer Res 61(8):3508–3517
Trevino JG et al (2005) Expression and activity of SRC regulate interleukin-8 expression in pancreatic adenocarcinoma cells: implications for angiogenesis. Cancer Res 65(16):7214–7222
Summy JM et al (2005) c-Src regulates constitutive and EGF-mediated VEGF expression in pancreatic tumor cells through activation of phosphatidyl inositol-3 kinase and p38 MAPK. Pancreas 31(3):263–274
Frame MC (2002) Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta 1602(2):114–130
Hakam A et al (2003) Coexpression of IGF-1R and c-Src proteins in human pancreatic ductal adenocarcinoma. Dig Dis Sci 48(10):1972–1978
Lutz MP et al (1998) Overexpression and activation of the tyrosine kinase Src in human pancreatic carcinoma. Biochem Biophys Res Commun 243(2):503–508
Morton JP et al (2010) Dasatinib inhibits the development of metastases in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology 139(1):292–303
Duxbury MS et al (2004) Inhibition of SRC tyrosine kinase impairs inherent and acquired gemcitabine resistance in human pancreatic adenocarcinoma cells. Clin Cancer Res 10(7):2307–2318
Ito H et al (2003) Inhibition of tyrosine kinase Src suppresses pancreatic cancer invasiveness. Surgery 134(2):221–226
Duong HQ et al (2014) Combination of dasatinib and gemcitabine reduces the ALDH1A1 expression and the proliferation of gemcitabine-resistant pancreatic cancer MIA PaCa-2 cells. Int J Oncol 44(6):2132–2138
Yezhelyev MV et al (2004) Inhibition of SRC tyrosine kinase as treatment for human pancreatic cancer growing orthotopically in nude mice. Clin Cancer Res 10(23):8028–8036
Trevino JG et al (2006) Inhibition of SRC expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. Am J Pathol 168(3):962–972
Bromann PA et al (2004) The interplay between Src family kinases and receptor tyrosine kinases. Oncogene 23(48):7957–7968
Ishizawar R, Parsons SJ (2004) c-Src and cooperating partners in human cancer. Cancer Cell 6(3):209–214
Biscardi JS et al (2000) Tyrosine kinase signalling in breast cancer: epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Res 2(3):203–210
Nagaraj NS et al (2011) Combined blockade of Src kinase and epidermal growth factor receptor with gemcitabine overcomes STAT3-mediated resistance of inhibition of pancreatic tumor growth. Clin Cancer Res 17(3):483–493
Lockhart AC et al (2003) A clinical model of dermal wound angiogenesis. Wound Repair Regen 11(4):306–313
Jia J et al (2015) Direct evidence of target inhibition with Anti-VEGF, EGFR, and mTOR therapies in a clinical model of wound healing. Clin Cancer Res 21(15):3442–3452
Chee CE et al (2013) Phase II study of dasatinib (BMS-354825) in patients with metastatic adenocarcinoma of the pancreas. Oncologist 18(10):1091–1092
Hong DS et al (2013) A phase 1 study of gemcitabine combined with dasatinib in patients with advanced solid tumors. Invest New Drugs 31(4):918–926
Argiris A et al (2012) Phase I and pharmacokinetic study of dasatinib and cetuximab in patients with advanced solid malignancies. Invest New Drugs 30(4):1575–1584
Dvorak HF (1986) Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315(26):1650–1659
Lockhart AC et al (2003) Reduction of wound angiogenesis in patients treated with BMS-275291, a broad spectrum matrix metalloproteinase inhibitor. Clin Cancer Res 9(2):586–593
Secord AA et al (2014) Dasatinib (BMS-35482) interacts synergistically with docetaxel, gemcitabine, topotecan, and doxorubicin in ovarian cancer cells with high SRC pathway activation and protein expression. Int J Gynecol Cancer 24(2):218–225
Sunada H et al (1990) Modulation of tyrosine, serine, and threonine phosphorylation and intracellular processing of the epidermal growth factor receptor by antireceptor monoclonal antibody. J Cell Physiol 142(2):284–292
Garrett TP et al (2003) The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell 11(2):495–505
Pan Y et al (2015) A preclinical evaluation of SKLB261, a multikinase inhibitor of EGFR/Src/VEGFR2, as a therapeutic agent against pancreatic cancer. Mol Cancer Ther 14(2):407–418
Naing A et al (2013) A phase I trial of KX2-391, a novel non-ATP competitive substrate-pocket- directed SRC inhibitor, in patients with advanced malignancies. Invest New Drugs 31(4):967–973
Renouf DJ et al (2012) A phase I/II study of the Src inhibitor saracatinib (AZD0530) in combination with gemcitabine in advanced pancreatic cancer. Invest New Drugs 30(2):779–786
Evans TRJ et al (2017) Phase 2 placebo-controlled, double-blind trial of dasatinib added to gemcitabine for patients with locally-advanced pancreatic cancer. Ann Oncol 28(2):354–361. https://doi.org/10.1093/annonc/mdw607
Deharvengt S et al (2012) Concomitant targeting of EGF receptor, TGF-beta and SRC points to a novel therapeutic approach in pancreatic cancer. PLoS One 7(6):e39684
Gomes EG et al (2013) Targeting the yin and the yang: combined inhibition of the tyrosine kinase c-Src and the tyrosine phosphatase SHP-2 disrupts pancreatic cancer signaling and biology in vitro and tumor formation in vivo. Pancreas 42(5):795–806
Strickler JH et al (2014) Phase I study of dasatinib in combination with capecitabine, oxaliplatin and bevacizumab followed by an expanded cohort in previously untreated metastatic colorectal cancer. Invest New Drugs 32(2):330–339
Arcaroli J et al (2012) Biomarker-driven trial in metastatic pancreas cancer: feasibility in a multicenter study of saracatinib, an oral Src inhibitor, in previously treated pancreatic cancer. Cancer Med 1(2):207–217
Acknowledgements
We gratefully acknowledge the participation of the patients and their caregivers in this study. We would like to acknowledge the Duke University GI Oncology Clinical Trials Team.
Funding
This was an investigator-initiated study supported by Bristol Myers Squib, Inc. The study was independently managed and analyzed. The final responsibility for the manuscript and the decision to submit for publication was made by the investigators. This work was also supported by National Institute of Health Grant 5K24-CA113755-05 (HH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
NBM is a clinical investigator on clinical trials supported by Incyte, ARMO, OncoMed, and Genentech/Roche. HU is a clinical investigator on clinical trials supported by Macrogenics, Merck, Genentech/Roche. ABN reports honoraria for consulting and advisory boards for Eli Lilly, Pfizer, and Kanghong Pharma. He has received grant support from Acceleron Pharma, Amgen, AstraZeneca/MedImmune, Eureka Therapeutics, Genentech, Leadiant Biosciences, MedPacto Inc, Novartis, Seattle Genetics, and Tracon Pharma. HH is employed by Genentech/Roche. The remaining authors have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mettu, N.B., Niedzwiecki, D., Rushing, C. et al. A phase I study of gemcitabine + dasatinib (gd) or gemcitabine + dasatinib + cetuximab (GDC) in refractory solid tumors. Cancer Chemother Pharmacol 83, 1025–1035 (2019). https://doi.org/10.1007/s00280-019-03805-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03805-6