Summary
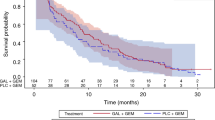
Aim This phase I/II study of saracatinib in combination with gemcitabine in patients with advanced pancreatic cancer was conducted by the NCIC Clinical Trials Group. The aims were to define the recommended phase II dose (RP2D) of saracatinib when combined with gemcitabine, and assess the efficacy of this combination in advanced pancreatic cancer. Patients and Methods Eligibility criteria included locally advanced or metastatic pancreatic adenocarcinoma and no prior chemotherapy. In phase I saracatinib was escalated in combination with gemcitabine (1000 mg/m2) to determine the recommended phase II dose (RP2D). The study was then expanded to a single arm phase II trial using a Simon 2-stage design. The primary endpoint was objective tumor response (OR) plus stable disease ≥4 months (SD4) rate; if ≥8 patients had OR+SD4, the study would proceed to stage 2. Results Thirteen patients were enrolled into the phase I portion of this study. Saracatinib 175 mg PO daily was chosen as the RP2D in combination with gemcitabine. Twenty-one additional patients were then enrolled at the RP2D (phase II). Of the 22 response evaluable patients treated at the RP2D, 9 patients (40.9%) had progressive disease, 6 patients (27.3%) had stable disease for less than 4 months, 5 patients (22.7%) had SD4, and 2 patients (9.1%) had a partial response to treatment. Objective criteria for continuing to stage 2 were thus not met and the trial was closed following the accrual of 34 patients. Conclusion Saracatinib 175 mg daily in combination with gemcitabine is well tolerated but the combination did not improve efficacy over what would be expected from gemcitabine alone.


Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ (2008) Cancer statistics, 2008. CA Cancer J Clin. doi:10.3322/CA.2007.0010
Glimelius B, Hoffman K, Sjoden PO, Jacobsson G, Sellstrom H, Enander LK, Linne T, Svensson C (1996) Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W, National Cancer Institute of Canada Clinical Trials Group (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. doi:10.1200/JCO.2006.07.9525
Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol
Yip D, Karapetis C, Strickland A, Steer CB, Goldstein D (2006) Chemotherapy and radiotherapy for inoperable advanced pancreatic cancer. Cochrane Database Syst Rev. doi:10.1002/14651858.CD002093.pub2
Martin GS (2001) The hunting of the Src. Nat Rev Mol Cell Biol. doi:10.1038/35073094
Boyer B, Bourgeois Y, Poupon MF (2002) Src kinase contributes to the metastatic spread of carcinoma cells. Oncogene. doi:10.1038/sj.onc.1205298
Lutz MP, Esser IB, Flossmann-Kast BB, Vogelmann R, Luhrs H, Friess H, Buchler MW, Adler G (1998) Overexpression and activation of the tyrosine kinase Src in human pancreatic carcinoma. Biochem Biophys Res Commun. doi:10.1006/bbrc.1997.8043
Hennequin LF, Allen J, Breed J, Curwen J, Fennell M, Green TP, Lambert-van der Brempt C, Morgentin R, Norman RA, Olivier A, Otterbein L, Ple PA, Warin N, Costello G (2006) N-(5-chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methylpiperazin-1-yl)ethoxy]-5- (tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine, a novel, highly selective, orally available, dual-specific c-Src/Abl kinase inhibitor. J Med Chem. doi:10.1021/jm060434q
Tabernero J, Cervantes A, Hoekman K et al (2007) Phase I study of AZD0530, an oral potent inhibitor of Src kinase: First demonstration of inhibition of Src activity in human cancers. J Clin Oncol 25:Abst 3520
Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE (2004) Inhibition of SRC tyrosine kinase impairs inherent and acquired gemcitabine resistance in human pancreatic adenocarcinoma cells. Clin Cancer Res
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Controlled Clinical Trials 1–10
Moore MJ, Hamm J, Dancey J, Eisenberg PD, Dagenais M, Fields A, Hagan K, Greenberg B, Colwell B, Zee B, Tu D, Ottaway J, Humphrey R, Seymour L, National Cancer Institute of Canada Clinical Trials Group (2003) Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. doi:10.1200/JCO.2003.02.098
Zee B, Melnychuk D, Dancey J, Eisenhauer E (1999) Multinomial phase II cancer trials incorporating response and early progression. J Biopharm Stat
Dent S, Zee B, Dancey J, Hanauske A, Wanders J, Eisenhauer E (2001) Application of a new multinomial phase II stopping rule using response and early progression. J Clin Oncol
Karrison TG, Maitland ML, Stadler WM, Ratain MJ (2007) Design of phase II cancer trials using a continuous endpoint of change in tumor size: application to a study of sorafenib and erlotinib in non small-cell lung cancer. J Natl Cancer Inst. doi:10.1093/jnci/djm158
Dhani N, Tu D, Sargent DJ, Seymour L, Moore MJ (2009) Alternate endpoints for screening phase II studies. Clin Cancer Res. doi:10.1158/1078-0432.CCR-08-2034
Price TJ, Lipton L, McGreivy J, McCoy S, Sun YN, Rosenthal MA (2008) Safety and pharmacokinetics of motesanib in combination with gemcitabine for the treatment of patients with solid tumours. Br J Cancer. doi:10.1038/sj.bjc.6604723
Jimeno A, Solomon A, Karikari C et al (2008) A prospective validation of a direct tumor xenograft model in pancreatic ductal adenocarcinoma (PDA). J Clin Oncol 26:Abstr 4500
Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW (2008) Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. doi:10.1126/science.1164368
Rajeshkumar NV, Tan AC, De Oliveira E, Womack C, Wombwell H, Morgan S, Warren MV, Walker J, Green TP, Jimeno A, Messersmith WA, Hidalgo M (2009) Antitumor effects and biomarkers of activity of AZD0530, a Src inhibitor, in pancreatic cancer. Clin Cancer Res. doi:10.1158/1078-0432.CCR-08-3021
Funding
This study was supported by funding from the Canadian Cancer Society. AstraZeneca provided saracatinib and partial funding to support the study.
Conflicts of Interest
Lesley Seymour received research funding from Astra Zeneca for this trial and has shares with Astra Zeneca. Derek Jonker attended an advisory board for Astra Zeneca.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Renouf, D.J., Moore, M.J., Hedley, D. et al. A phase I/II study of the Src inhibitor saracatinib (AZD0530) in combination with gemcitabine in advanced pancreatic cancer. Invest New Drugs 30, 779–786 (2012). https://doi.org/10.1007/s10637-010-9611-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-010-9611-3