Abstract
Purpose
Intestinal mucositis and diarrhea are common manifestations of anticancer regimens that include irinotecan, 5-fluorouracil (5-FU), and other cytotoxic drugs. These side effects negatively impact therapeutic outcomes and delay subsequent cycles of chemotherapy, resulting in dose reductions and treatment discontinuation. Here, we aimed to review the experimental evidence regarding possible new targets for the management of irinotecan- and 5-FU-related intestinal mucositis.
Methods
A literature search was performed using the PubMed and MEDLINE databases. No publication time limit was set for article inclusion.
Results
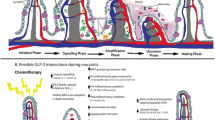
Here, we found that clinical management of intestinal mucositis and diarrhea is somewhat ineffective at reducing symptoms, possibly due to a lack of specific targets for modulation. We observed that IL-1β contributes to the apoptosis of enterocytes in mucositis induced by 5-FU. However, 5-FU-related mucositis is far less thoroughly investigated with regard to specific molecular targets when compared to irinotecan-related disease. Several studies have proposed that a correlation exists between the intestinal microbiota, the enterohepatic recirculation of active metabolites of irinotecan, and the establishment of mucositis. However, as reviewed here, this association seems to be controversial. In addition, the pathogenesis of irinotecan-induced mucositis appears to be orchestrated by interleukin-1/Toll-like receptor family members, leading to epithelial cell apoptosis.
Conclusions
IL-1β, IL-18, and IL-33 and the receptors IL-1R, IL-18R, ST2, and TLR-2 are potential therapeutic targets that can be modulated to minimize anticancer agent-associated toxicity, optimize cancer treatment dosing, and improve clinical outcomes. In this context, the pathogenesis of mucositis caused by other anticancer agents should be further investigated.

Similar content being viewed by others
Abbreviations
- ASC :
-
Inflammasome adaptor protein apoptosis-associated speck-like protein containing CARD
- CCL :
-
Chemokine (C–C motif) Ligand
- CID :
-
Chemotherapy-induced diarrhea
- COX-2 :
-
Cyclooxygenase-2
- CPT-11 :
-
Irinotecan
- CXCL :
-
Chemokine (C–X–C motif) ligand
- CXCR :
-
Chemokine (C–X–C Motif) receptor
- DAMP :
-
Damage-associated molecular pattern
- DC :
-
Dendritic cell
- FOLFIRI :
-
Irinotecan with fluorouracil and folinic acid
- IL-18 :
-
Interleukin-18
- IL-18R :
-
Interleukin-18 receptor
- IL-1R :
-
Interleukin-1 receptor
- IL-1RA :
-
Interleukin-1 receptor antagonist
- IL-1β :
-
Interleukin-1β
- IL-33 :
-
Interleukin-33
- iNOS :
-
Inducible nitric oxide synthase
- IROX :
-
Irinotecan and oxaliplatin
- MyD88 :
-
Myeloid differentiation primary response gene 88
- NADPH :
-
Dihydronicotinamide adenine dinucleotide phosphate
- NF-κB:
-
Nuclear factor kappa B
- NO :
-
Nitric oxide
- NOX-2 :
-
NADPH oxidase-2
- PAMP :
-
Pathogen-associated molecular pattern
- ROS :
-
Reactive oxygen species
- 5-FU:
-
5-Fluorouracil
- SN-38 :
-
7-Ethyl-10-hydroxycamptothecin or the active metabolite of irinotecan
- SN-38G :
-
SN-38 glucuronide form
- ST2 :
-
Interleukin-33 receptor
- TIR :
-
Toll/IL-1 receptor domain
- TLR :
-
Toll-like receptor
- TNF-α :
-
Tumor necrosis factor-α
- UGT1A1 :
-
UDP glucuronosyltransferase 1 family, polypeptide A1
References
Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE et al (2007) Mucositis study section of the multinational association of supportive care in cancer and the international society for oral oncology. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 109:820–831
Duncan M, Grant G (2003) Oral and intestinal mucositis—causes and possible treatments. Aliment Pharmacol Ther 18:853–874
Jones JA, Avritscher EB, Cooksley CD, Michelet M, Bekele BN, Elting LS (2006) Epidemiology of treatment-associated mucosal injury after treatment with newer regimens for lymphoma, breast, lung, or colorectal cancer. Support Care Cancer 14(6):505–515
Dranitsaris G, Maroun J, Shah A (2005) Severe chemotherapy-induced diarrhea in patients with colorectal cancer: a cost of illness analysis. Support Care Cancer 13:318–324
Rubenstein EB, Peterson DE, Schubert M, Keefe D, McGuire D, Epstein J et al (2004) Mucositis study section of the multinational association for supportive care in cancer; international society for oral oncology. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 100(9 Suppl):2026–2046
U.S. Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE), version 4.03, June 2010, National Institutes of Health, National Cancer Institute. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed 22 Oct 2015
Avallone A, Di Gennaro E, Silvestro L, Laffaioli VR, Budillon A (2014) Targeting thymidylate synthase in colorectal cancer: critical re-evaluation and emerging therapeutic role of raltitrexed. Expert Opin Drug Saf 2014(13):113–129
Soares PGM, Mota JMSC, Gomes AS, Oliveira RB, Assreuy AMS, Brito GAC et al (2008) Gastrointestinal dysmotility in 5-fluorouracil-induced intestinal mucositis outlasts inflammatory process resolution. Cancer Chemother Pharmacol 63:91–98
Logan RM, Stringer AM, Bowen JM, Gibson RJ, Sonis ST, Keefe DM (2009) Is the pathobiology of chemotherapy-induced alimentary tract mucositis influenced by the type of mucotoxic drug administered? Cancer Chemother Pharmacol 63:239–251
Chang CT, Ho TY, Lin H, Liang JA, Huang HC et al (2012) 5-fluorouracil induced intestinal mucositis via nuclear factor-kB activation by transcriptomic analysis and in vivo bioluminescence imaging. PLoS ONE 7(3):e31808. doi:10.1371/journal.pone.0031808
Yasuda M, Kato S, Yamanaka N, Iimori M, Utsumi D, Kitahara Y et al (2012) Potential role of the NADPH oxidase NOX1 in the pathogenesis of 5-fluorouracil-induced intestinal mucositis in mice. Am J Physiol Gastrointest Liver Physiol 302:G1133–G1142
Wu Z, Han X, Wang Y, Yuan K, Jin Z, Di J et al (2011) Interleukin-1 receptor antagonist reduced apoptosis and attenuated intestinal mucositis in a 5-Xuorouracil chemotherapy model in mice. Cancer Chemother Pharmacol 68:87–96
Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ et al (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343:905–914
Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K (1991) Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res 51:4187–4191
Dancey J, Eisenhauer EA (1996) Current perspectives on camptothecins in cancer treatment. Br J Cancer 74:327–338
Ma MK, McLeod HL (2003) Lessons learned from the irinotecan metabolic pathway. Curr Med Chem 10:41–49
Saltz LB, Douillard JY, Pirotta N, Alakl M, Gruia G, Awad L et al (2001) Irinotecan plus fluorouracil/leucovorin for metastatic colorectal cancer: a new survival standard. Oncologist 6:81–91
Ohno R, Okada K, Masaoka T, Kuramoto A, Arima T, Yoshida Y et al (1990) An early phase II study of CPT-11: a new derivative of camptothecin, for the treatment of leukemia and lymphoma. J Clin Oncol 8:1907–1912
Stein A, Voigt W, Jordan K (2010) Chemotherapy-induced diarrhea: pathophysiology, frequency and guideline-based management. Ther Adv Med Oncol 2:51–63
Andreyev J, Ross P, Donnellan C, Lennan E, Leonard P, Waters C et al (2014) Guidance on the management of diarrhoea during cancer chemotherapy. Lancet Oncol 15:e447–e460
Araki E, Ishikawa M, Iigo M, Koide T, Itabashi M, Hoshi A (1993) Relationship between development of diarrhea and the concentration of SN-38, an active metabolite of CPT-11, in the intestine and the blood plasma of athymic mice following intraperitoneal administration of CPT-11. Jpn J Cancer Res 84:697–702
Melo MLP, Brito GAC, Soares RC, Carvalho SB, Silva JV, Soares PM et al (2008) Role of cytokines (TNF-a, IL-1b and KC) in the pathogenesis of CPT-11-induced intestinal mucositis in mice: effect of pentoxifylline and thalidomide. Cancer Chemother Pharmacol 61:775–784
Dodds HM, Rivory LP (1999) The mechanism for the inhibition of acetylcholinesterases by irinotecan (CPT-11). Mol Pharmacol 56:1346–1353
Morton CL, Wadkins RM, Danks MK, Potter PM (1999) The anticancer prodrug CPT-11 is a potent inhibitor of acetylcholinesterase but is rapidly catalyzed to SN-38 by butyrylcholinesterase. Cancer Res 59:1458–1463
Blandizzi C, De Paolis B, Colucci R, Lazzeri G, Baschiera F, Del Tacca M (2001) Characterization of a novel mechanism accounting for the adverse cholinergic effects of the anticancer drug irinotecan. Br J Pharmacol 132:73–84
Blandizzi D, Danesi R, Paolis B, Di Paolo A, Colucci R, Falcone A et al (2002) Cholinergic toxic syndrome by the anticancer drug irinotecan: acetylcholinesterase does not play a major role. Clin Pharmacol Ther 71:263–271
Hecht R (1998) Gastrointestinal toxicity of irinotecan. Oncology 12:73–78
Lima-Júnior RCP, Figueiredo AA, Freitas HC, Melo ML, Wong DV, Leite CA et al (2012) Involvement of nitric oxide on the pathogenesis of irinotecan-induced intestinal mucositis: role of cytokines on inducible nitric oxide synthase activation. Cancer Chemother Pharmacol 69:931–942
Lima-Júnior RCP, Freitas HC, Wong DVT, Wanderley CW, Nunes LG, Leite LL et al (2014) Targeted inhibition of IL-18 attenuates irinotecan induced intestinal mucositis in mice. Br J Pharmacol 171:2335–2350
Wong DV, Lima-Júnior RC, Carvalho CB, Borges VF, Wanderley CW, Bem AX et al (2015) The adaptor protein Myd88 is a key signaling molecule in the pathogenesis of irinotecan-induced intestinal mucositis. PLoS ONE 10:e0139985
Puduvalli VK, Giglio P, Groves MD, Hess KR, Gilbert MR, Mahankali S et al (2008) Phase II trial of irinotecan and thalidomide in adults with recurrent glioblastoma multiforme. Neuro Oncol 10:216–222
Takasuna K, Hagiwara T, Hirohashi M, Kato M, Nomura M, Nagai E et al (1998) Inhibition of intestinal microflora beta-glucuronidase modifies the distribution of the active metabolite of the antitumor agent, irinotecan hydrochloride (CPT-11) in rats. Cancer Chemother Pharmacol 42:280–286
Itoh T, Takemoto I (2004) Biliary excretion of irinotecan and its metabolites. J Pharm Pharm Sci 7:13–18
Takasuna KTH, Hirohashi M, Kato M, Kato M, Nomura M, Nagai E et al (1996) Involvement of β-glucuronidase in intestinal microflora in the intestinal toxicity of the antitumor camptothecin derivative irinotecan hydrochloride (CPT-11) in rats. Cancer Res 56:3752–3757
Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh AS, Keefe DM (2008) Faecal microflora and β-glucuronidase expression are altered in an irinotecan-induced diarrhea model in rats. Cancer Biol Ther 7:1919–1925
Sezer A, Ust U, Cicin I (2009) The effect of Saccharomyces boulardii on reducing irinotecan-induced intestinal mucositis and diarrhea. Med Oncol 26:350–357
Roberts AB, Wallace BD, Venkatesh MK, Mani S, Redinbo MR et al (2013) Molecular insights into microbial β-glucoronidase inhibition to abrogate CPT-11 toxicity. Mol Pharmacol 84:208–217
Kehrer DFC, Sparreboom A, Verweij J, de Bruijn P, Nierop CA, van de Schraaf J et al (2001) Modulation of irinotecan-induced diarrhea by cotreatment with neomycin in cancer patients. Clin Cancer Res 7:1136–1141
Kurita A, Kado S, Matsumoto T, Asakawa N, Kaneda N, Kato I et al (2011) Streptomycin alleviates irinotecan-induced delayed-onset diarrhea in rats by a mechanism other than inhibition of β-glucuronidase activity in intestinal lumen. Cancer Chemother Pharmacol 67:201–213
Pedroso SH, Vieira AT, Bastos RW, Oliveira JS, Cartelle CT, Arantes RM et al (2015) Evaluation of mucositis induced by irinotecan after microbial colonization in germ-free mice. Microbiology 161:1950–1960
Brandi G, Dabard J, Raibaud P, Di Battista M, Bridonneau C, Pisi AM et al (2006) Intestinal microflora and digestive toxicity of irinotecan in mice. Clin Cancer Res 12:1299–1307
Ikuno N, Soda H, Watanabe M, Oka M (1995) Irinotecan (CPT-11) and characteristic mucosal changes in the mouse ileum and cecum. J Natl Cancer Inst 87(24):1876–1883
Sparreboom A, De Jonge MJ, De Bruijn P, Brouwer E, Nooter K, Loos WJ et al (1998) Irinotecan (CPT-11) metabolism and disposition in cancer patients. Clin Cancer Res 4:2747–2754
Ulukan H, Muller MT, Swaan PW (2001) Downregulation of topoisomerase I in differentiating human intestinal epithelial cells. Int J Cancer 94:200–207
Bowen JM, Gibson RJ, Cummins AG (2006) Intestinal mucositis: the role of the Bcl-2 family, p53 and caspases in chemotherapy-induced damage. Support Care Cancer 14:713–731
Tsujimoto Y (1998) Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells 3:697–707
Besbes S, Mirshahi M, Pocard M, Billard C (2015) New dimension in therapeutic targeting of BCL-2 family proteins. Oncotarget 30:12862–12871
Krajewski S, Krajewska M, Shabaik A, Miyashita T, Wang HG, Reed JC (1994) Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am J Pathol 145:1323–1336
Bowen JM, Gibson RJ, Tsykin A, Stringer AM, Logan RM, Keefe DM (2007) Gene expression analysis of multiple gastrointestinal regions reveals activation of common cell regulatory pathways following cytotoxic chemotherapy. Int J Cancer 121:1847–1856
Bowen JM, Tsykin A, Stringer AM, Logan RM, Gibson RJ, Keefe DM (2010) Kinetics and regional specificity of irinotecan-induced gene expression in the gastrointestinal tract. Toxicology 269:1–12
Czabotar PE, Lessene G, Strasser A, Adams JM (2014) Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 15:49–63
Bowen JM, Gibson RJ, Stringer AM, Chan TW, Prabowo AS, Cummins AG et al (2007) Role of p53 in irinotecan-induced intestinal cell death and mucosal damage. Anticancer Drugs 18:197–210
McIlwain DR, Berger T, Mak TW (2013) Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 5:a008656
Chowdhury I, Tharakan B, Bhat GK (2008) Caspases—an update. Comp Biochem Physiol 151:10–27
Arifa RDN, Madeira MF, de Paula TP, Lima RL, Tavares LD, Menezes-Garcia Z et al (2014) Inflammasome activation is reactive oxygen species dependent and mediates irinotecan-induced mucositis through IL-1b and IL-18 in mice. Am J Pathol 184:2023–2034
Wang X, Zhub S, Qiana L, Gao J, Wu M, Gao J et al (2014) IL-1Ra selectively protects intestinal crypt epithelial cells, but not tumor cells, from chemotoxicity via p53-mediated upregulation of p21WAF1and p27KIP1. Pharmacol Res 82:21–33
Guabiraba R, Besnard AG, Menezes GB, Secher T, Jabir MS, Amaral SS et al (2014) IL-33 targeting attenuates intestinal mucositis and enhances effective tumor chemotherapy in mice. Mucosal Immunol 7:1079–1093
Bohnenkamp HR, Papazisis KT, Burchell JM, Taylor-Papadimitriou J (2007) Synergism of toll-like receptor-induced interleukin-12p70 secretion by monocyte-derived dendritic cells is mediated through p38 MAPK and lowers the threshold of T-helper cell type 1 responses. Cell Immunol 247:72–84
Frosali S, Pagliari D, Gambassi G, Landolfi R, Pandolfi F, Cianci R (2015) How the intricate interaction among toll-like receptors, microbiota, and intestinal immunity can influence gastrointestinal pathology. J Immunol Res 2015:489821
Chen K, Huang J, Gong W, Iribarren P, Dunlop NM, Wang JM (2007) Toll-like receptors in inflammation, infection and cancer. Int Immunopharmacol 7:1271–1285
Bianchi ME (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81:1–5
Akira S, Takeda K, Kaisho T (2001) Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2:675–680
Takeda K, Akira S (2004) TLR signaling pathways. Semin Immunol 16:3–9
Fernandes P, MacSharry J, Darby T, Fanning A, Shanahan F, Houston A et al (2015) Differential expression of key regulators of toll-like receptors in ulcerative Colitis and Crohn’s disease: a role for Tollip and PPARγ. Clin Exp Immunol. doi:10.1111/cei.12732
Fan Y, Liu B (2015) Expression of toll-like receptors in the mucosa of patients with ulcerative colitis. Exp Ther Med 9:1455–1459
Lu CC, Kuo HC, Wang FS, Jou MH, Lee KC, Chuang JH (2014) Upregulation of TLRs and IL-6 as a marker in human colorectal cancer. Int J Mol Sci 16:159–177
Kaczmarek A, Brinkman BM, Heyndrickx L, Vandenabeele P, Krysko DV (2012) Severity of doxorubicin-induced small intestinal mucositis is regulated by the TLR-2 and TLR-9 pathways. J Pathol 226:598–608
Frank M, Hennenberg EM, Eyking A, Rünzi M, Gerken G, Scott P et al (2015) TLR signaling modulates side effects of anticancer therapy in the small intestine. J Immunol 194:1983–1995
Ribeiro RA, Lima-Junior RC, Leite CAV, Mota JMS, Macedo FY, Lima MV et al (2012) Chemotherapy-induced hemorrhagic cystitis: pathogenesis, pharmacological approaches and new insights. J Exp Integr Med 2:95–112
Trifan OC, Durham WF, Salazar VS, Horton J, Levine BD, Zweifel BS et al (2002) Cyclooxygenase-2 inhibition with celecoxib enhances antitumor efficacy and reduces diarrhea side effect of CPT-11. Cancer Res 62:5778–5784
Javle MM, Cao S, Durrani FA, Pendyala L, Lawrence DD, Smith PF et al (2007) Celecoxib and mucosal protection: translation from an animal model to a phase I clinical trial of celecoxib, irinotecan, and 5-fluorouracil. Clin Cancer Res 13:965–971
Leitão RF, Ribeiro RA, Bellaguarda EA, Macedo FD, Silva LR, Oriá RB et al (2007) Role of nitric oxide on pathogenesis of 5-fluorouracil induced experimental oral mucositis in hamster. Cancer Chemother Pharmacol 59:603–612
Leitão RF, Brito GA, Oriá RB, Braga-Neto MB, Bellaguarda EA, Silva JV et al (2011) Role of inducible nitric oxide synthase pathway on methotrexate-induced intestinal mucositis in rodents. BMC Gastroenterol 16:11–90
Van Sebille YZ, Stansborough R, Wardill HR, Bateman E, Gibson RJ, Keefe DM (2015) Management of mucositis during chemotherapy: from pathophysiology to pragmatic therapeutics. Curr Oncol Rep 17:50
Krishnamurthi SS Enterotoxicity of chemotherapeutic agents. UPTODATE. [Online] www.uptodate.com/contents/enterotoxicity-of-chemotherapeutic-agents. Accessed 20 Oct 2015
Peterson DE, Bensadoun RJ, Roila F, ESMO Guidelines Working Group (2009) Management of oral and gastrointestinal mucositis: ESMO clinical recommendations. Ann Oncol 20:174–177
Ychou M, Douillard JY, Rougier P, Adenis A, Mousseau M, Dufour P et al (2000) Randomized comparison of prophylactic antidiarrheal treatment versus no prophylactic antidiarrheal treatment in patients receiving CPT-11 (irinotecan) for advanced 5-FU-resistant colorectal cancer: an open-label multicenter phase II study. Am J Clin Oncol 23:143–148
Karthaus M, Ballo H, Abenhardt W, Steinmetz T, Geer T, Schimke J et al (2005) Prospective, double-blind, placebo-controlled, multicenter, randomized phase III study with orally administered budesonide for prevention of irinotecan (CPT-11)-induced diarrhea in patients with advanced colorectal cancer. Oncology 68:326–332
Gibson RJ, Bowen JM, Keefe DMK (2005) Palifermin reduces diarrhea and increases survival following irinotecan treatment in tumor-bearing DA rats. Int J Cancer 116:464–470
Heel RC, Brogden RN, Speight TM, Avery GS (1978) Loperamide: a review of its pharmacological properties and therapeutic efficacy in diarrhoea. Drugs 15:33–52
Sharma R, Tobin P, Clarke SJ (2005) Management of chemotherapy-induced nausea, vomiting, oral mucositis, and diarrhoea. Lancet Oncol 6:93–102
Regnard C, Twycross R, Mihalyo M, Wilcock A (2011) Loperamide. J Pain Symptom Manag 42:319–323
Ibhanesebhor O Review of the role of loperamide and codeine in the management of symptomatic diarrhoea in adults. [Online] http://www.who.int/selection_medicines/committees/expert/18/applications/LoperamideCodeine.pdf. Accessed 20 July 2015
Conti JA, Kemeny NE, Saltz LB, Huang Y, Tong WP, Chou TC et al (1996) Irinotecan is an active agent in untreated patients with metastatic colorectal cancer. J Clin Oncol 14:709–715
Vamvakas L, Kakolyris S, Kouroussis C, Kandilis K, Mavroudis D, Ziras N, Greek Colorectal Cooperative Oncology Group. et al (2002) Irinotecan (CPT-11) in combination with infusional 5-fluorouracil and leucovorin (de Gramont regimen) as first-line treatment in patients with advanced colorectal cancer: a multicenter phase II study. Am J Clin Oncol 25:65–70
Sun L, Coy DH (2014) Somatostatin and its analogs. Curr Drug Targets. [Epub ahead of print]
Sun JX, Yang N (2014) Role of octreotide in post chemotherapy and/or radiotherapy diarrhea: prophylaxis or therapy? Asia Pac J Clin Oncol 10:e108–e113
Ruskone A, Rene E, Chayvialle JA, Bonin N, Pignal F, Kremer M et al (1982) Effect of soma-tostatin on diarrhea and on small intestinal water and electrolyte transport in a patient with pancreatic cholera. Dig Dis Sci 27:459–466
Barbounis V, Koumakis G, Vassilomanolakis M, Demiri M, Efremidis AP (2001) Control of irinotecan-induced diarrhea by octreotide after loperamide failure. Support Care Cancer 9:258–260
Bhattacharya S, Vijayasekar C, Worlding J, Mathew G (2009) Octreotide in chemotherapy induced diarrhoea in colorectal cancer: a review article. Acta Gastroenterol Belg 72:289–295
Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh AS, Burns J et al (2007) Chemotherapy-induced diarrhea is associated with changes in the luminal environment in the DA rat. Exp Biol Med 232:96–106
Schmittel A, Jahnke K, Thiel E, Keilholz U (2004) Neomycin as secondary prophylaxis for irinotecan-induced diarrhea. Ann Oncol 15:1296
Alimonti A, Satta F, Pavese I, Burattini E, Zoffoli V, Vecchione A (2003) Prevention of irinotecan plus 5-fluorouracil/leucovorin-induced diarrhoea by oral administration of neomycin plus bacitracin in first-line treatment of advanced colorectal cancer. Ann Oncol 14:805–806
Mani S, Boelsterli UA, Redinbo MR (2014) Understanding and modulating mammalian-microbial communication for improved human health. Annu Rev Pharmacol Toxicol 54:559–580
Pérez-Cobas AE, Gosalbes MJ, Friedrichs A, Knecht H, Artacho A, Eismann K et al (2013) Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut 62:1591–1601
Costa CL, Quesada-Gómez C, de Carvalho CB, González RH, Gifoni MA, Ribeiro RA et al (2014) Community-acquired diarrhea associated with clostridium difficile in an HIV-positive cancer patient: first case report in Latin America. Int J Infect Dis 26:138–139
Swami U, Goel S, Mani S (2013) Therapeutic targeting of CPT-11 induced diarrhea: a case for prophylaxis. Curr Drug Targets 14:777–797
Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S et al (2014) Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis—current evidence and potential clinical applications. Aliment Pharmacol Ther 40:409–421
Bowen JM, Stringer AM, Gibson RJ, Yeoh AS, Hannam S, Keefe DM (2007) VSL#3 probiotic treatment reduces chemotherapy-induced diarrhea and weight loss. Cancer Biol Ther 6:1449–1454
Osterlund P, Ruotsalainen T, Korpela R, Saxelin M, Ollus A, Valta P et al (2007) Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study. Br J Cancer 97:1028–1034
Wada M, Nagata S, Saito M, Shimizu T, Yamashiro Y, Matsuki T et al (2010) Effects of the enteral administration of Bifidobacterium breve on patients undergoing chemotherapy for pediatric malignancies. Support Care Cancer 18:751–759
Sakai H, Diener M, Gartmann V, Takeguchi N (1995) Eicosanoid-mediated Cl− secretion induced by the antitumor drug, irinotecan (CPT-11), in the rat colon. Naunyn-Schmiedeberg’s Arch Pharmacol 351:309–314
Cao S, Black JD, Troutt AB, Rustum YM (1998) Interleukin 15 offers selective protection from irinotecan-induced intestinal toxicity in a preclinical animal model. Cancer Res 58:3270–3274
Ikegami T, Ha L, Arimori K, Latham P, Kobayashi K, Ceryak S et al (2002) Intestinal alkalization as a possible preventive mechanism in irinotecan (CPT-11)-induced diarrhea. Cancer Res 62:179–187
Yang XX, Hu ZP, Xu AL, Duan W, Zhu YZ, Huang M et al (2006) A mechanistic study on reduced toxicity of irinotecan by coadministered thalidomide, a tumor necrosis factor-a inhibitor. J Pharmacol Exp Ther 319:82–104
Xue H, Sawyer MB, Field CJ, Dieleman LA, Baracos VE (2007) Nutritional modulation of antitumor efficacy and diarrhea toxicity related to irinotecan chemotherapy in rats bearing the ward colon tumor. Clin Cancer Res 13:7146-7154
Chen S, Yueha MF, Bigob C, Barbier O, Wang K, Karin M et al (2013) Intestinal glucuronidation protects against chemotherapy-induced toxicity by irinotecan (CPT-11). Proc Natl Acad Sci USA 110:19143–19148
Acknowledgments
This review is dedicated to the loving memory of Prof. Dr. Ronaldo Albuquerque Ribeiro (in memoriam). R. C. P. Lima-Júnior received a research Grant from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), Grant Nos.: 307143/2014-7 and 458872/2014-8. This work was also supported by Grants from CAPES (Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Grant CAPES-PROEX 2862/2013) and FUNCAP (Fundação Cearense de Apoio ao Desenvolvimento Científico, Grant PRONEX PR2-0101-00054.01.00/15).
Author’s contribution
RA. Ribeiro, C.W.S. Wanderley, D.V.T. Wong, J.M.S.C. Mota, C.A.V.G. Leite, M.H.L.P. Souza, F.Q. Cunha, and R.C.P. Lima-Júnior all contributed to the literature searching and reviewing and to the writing of the manuscript. C.A.V.G. Leite and C.W.S. Wanderley constructed the illustration and table, respectively. C.W.S. Wanderley, D.V.T. Wong, J.M.S.C. Mota, C.A.V.G. Leite, M.H.L.P. Souza, F.Q. Cunha, and R.C.P. Lima-Júnior approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ribeiro, R.A., Wanderley, C.W.S., Wong, D.V.T. et al. Irinotecan- and 5-fluorouracil-induced intestinal mucositis: insights into pathogenesis and therapeutic perspectives. Cancer Chemother Pharmacol 78, 881–893 (2016). https://doi.org/10.1007/s00280-016-3139-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3139-y