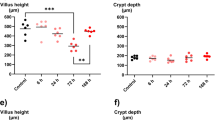
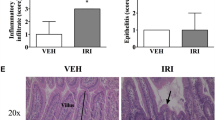
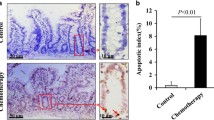
Abstract
Intestinal mucositis occurs as a consequence of cytotoxic treatment through multiple mechanisms including induction of crypt cell death (apoptosis) and cytostasis. The molecular control of these actions throughout the gastrointestinal tract has yet to be fully elucidated; however, they are known to involve p53, the Bcl-2 family and caspases. This review will provide an overview of current research as well as identify areas where gaps in knowledge exist.




Similar content being viewed by others
References
Adams JM, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281:1322–1326
Akgul C, Moulding DA, Edwards SW (2004) Alternative splicing of Bcl-2-related genes: functional consequences and potential therapeutic applications. Cell Mol Life Sci 61:2189–2199
Anilkumar TV, Sarraf CE, Hunt T, Alison MR (1992) The nature of cytotoxic drug-induced cell death in murine intestinal crypts. Br J Cancer 65:552–558
Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale F, Sadoul R, Martinou JC (1997) Inhibition of Bax channel-forming activity by Bcl-2. Science 277:370–372
Araki E, Ishikawa M, Iigo M, Koide T, Itabashi M, Hoshi A (1993) Relationship between development of diarrhea and the concentration of SN-38, an active metabolite of CPT-11, in the intestine and the blood plasma of athymic mice following intraperitoneal administration of CPT-11. Jpn J Cancer Res 84:697–702
Ashkenazi A, Dixit VM (1998) Death receptors: signaling and modulation. Science 281:1305–1308
Baker SJ, Markowitz S, Fearon ER, Willson JK, Vogelstein B (1990) Suppression of human colorectal carcinoma cell growth by wild-type p53. Science 249:912–915
Bartek J, Lukas J (2003) DNA repair: damage alert. Nature 421:486–488
Beck PL, Wong JF, Li Y, Swaminathan S, Xavier RJ, Devaney KL, Podolsky DK (2004) Chemotherapy- and radiotherapy-induced intestinal damage is regulated by intestinal trefoil factor. Gastroenterology 126:796–808
Benchimol S (2001) p53-dependent pathways of apoptosis. Cell Death Differ 8:1049–1051
Booth D, Potten CS (2001) Protection against mucosal injury by growth factors and cytokines. J Natl Cancer Inst Monographs 29:16–20
Borner C (2003) The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol Immunol 39:615–647
Boushey RP, Yusta B, Drucker DJ (2001) Glucagon-like peptide (GLP)-2 reduces chemotherapy-associated mortality and enhances cell survival in cells expressing a transfected GLP-2 receptor. Cancer Res 61:687–693
Bradham CA, Qian T, Streetz C, Trautwein C, Brenner DA, Lemasters JJ (1998) The mitochondrial permeability transition is required for tumor necrosis factor alpha-mediated apoptosis and cytochrome c release. Mol Cell Biol 18:6353–6364
Cao S, Black JD, Troutt AB, Rustum YM (1998) Interleukin 15 offers selective protection from irinotecan-induced intestinal toxicity in a preclinical animal model. Cancer Res 58:3270–3274
Catz SD, Johnson JL (2001) Transcriptional regulation of bcl-2 by nuclear factor kappa B and its significance in prostate cancer. Oncogene 20:7342–7351
Chao DT, Korsmeyer SJ (1998) BCL-2 family: regulators of cell death. Annu Rev Immunol 16:395–419
Chen C, Edelstein LC, Gelinas C (2000) The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L). Mol Cell Biol 20:2687–2695
Chernavsky AC, Rubio AE, Vanzulli S, Rubinstein N, de Rosa S, Fainboim L (2002) Evidences of the involvement of Bak, a member of the Bcl-2 family of proteins, in active coeliac disease. Autoimmunity 35:29–37
Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR (2004) Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303:1010–1014
Chu KU, Higashide S, Evers BM, Rajaraman S, Ishizuka J, Townsend CM, Thompson JC (1994) Bombesin improves survival from methotrexate-induced enterocolitis. Ann Surg 220:570–576; discussion 576–577
Cohen GM (1997) Caspases: the executioners of apoptosis. Biochem J 326:1–16
Cory S, Huang DC, Adams JM (2003) The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene 22:8590–8607
Creagh EM, Conroy H, Martin SJ (2003) Caspase-activation pathways in apoptosis and immunity. Immunol Rev 193:10–21
Crompton M (1999) The mitochondrial permeability transition pore and its role in cell death. Biochem J 341:233–249
Culmsee C, Zhu X, Yu QS, Chan SL, Camandola S, Guo Z, Greig NH, Mattson MP (2001) A synthetic inhibitor of p53 protects neurons against death induced by ischemic and excitotoxic insults, and amyloid beta-peptide. J Neurochem 77:220–228
Decary S, Decesse JT, Ogryzko V, Reed JC, Naguibneva I, Harel-Bellan A, Cremisi CE (2002) The retinoblastoma protein binds the promoter of the survival gene bcl-2 and regulates its transcription in epithelial cells through transcription factor AP-2. Mol Cell Biol 22:7877–7888
Decker-Baumann C, Buhl K, Frohmuller S, von Herbay A, Dueck M, Schlag PM (1999) Reduction of chemotherapy-induced side-effects by parenteral glutamine supplementation in patients with metastatic colorectal cancer. Eur J Cancer 35:202–207
DeLeo AB, Jay G, Appella E, Dubois GC, Law LW, Old LJ (1979) Detection of a transformation-related antigen in chemically induced sarcomas and other transformed cells of the mouse. Proc Natl Acad Sci U S A 76:2420–2424
Dippold WG, Jay G, DeLeo AB, Khoury G, Old LJ (1981) p53 transformation-related protein: detection by monoclonal antibody in mouse and human cells. Proc Natl Acad Sci U S A 78:1695–1699
Erster S, Mihara M, Kim RH, Petrenko O, Moll UM (2004). In vivo mitochondrial p53 translocation triggers a rapid first wave of cell death in response to DNA damage that can precede p53 target gene activation. Mol Cell Biol 24:6728–6741
Eskes R, Desagher S, Antonsson B, Martinou JC (2000) Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol 20:929–935
Fadeel B, Zhivotovsky B, Orrenius S (1999) All along the watchtower: on the regulation of apoptosis regulators. FASEB J 13:1647–1657
Finlay CA, HindsPW, Levine AJ (1989) The p53 proto-oncogene can act as a suppressor of transformation. Cell 57:1083–1093
Fleischer A, Rebollo A, Ayllon V (2003) BH3-only proteins: the lords of death. Arch Immunol Ther Exp 51:9–17
Foyouzi-Youssefi R, Arnaudeau S, Borner C, Kelley WL, Tschopp J, Lew DP, Demaurex N, Krause KH (2000) Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc Natl Acad Sci U S A 97:5723–5728
Funk MA, Baker DH (1991) Effect of soy products on methotrexate toxicity in rats. J Nutr 121:1684–1692
Gauthier R, Harnois C, Drolet JF, Reed JC, Vezina A, Vachon PH (2001) Human intestinal epithelial cell survival: differentiation state-specific control mechanisms. Am J Physiol Cell Physiol 280:C1540–C1554
Gauthier R, Laprise P, Cardin E, Harnois C, Plourde A, Reed JC, Vezina A, Vachon PH (2001) Differential sensitivity to apoptosis between the human small and large intestinal mucosae: linkage with segment-specific regulation of BCL-2 homologs and involvement of signaling pathways. J Cell Biochem 82:339–355
Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119:493–501
Ghribi O, DeWitt DA, Forbes MS, Herman MM, Savory J (2001) Co-involvement of mitochondria and endoplasmic reticulum in regulation of apoptosis: changes in cytochrome c, Bcl-2 and Bax in the hippocampus of aluminum-treated rabbits. Brain Res 903:66–73
Gibson LF, Fortney J, Magro G, Ericson SG, Lynch JP, Landreth KS (1999) Regulation of BAX and BCL-2 expression in breast cancer cells by chemotherapy. Breast Cancer Res Treat 55:107–117
Gibson RJ, Keefe DM, Clarke JM, Regester GO, Thompson FM, Goland GJ, Edwards BG, Cummins AG (2002) The effect of keratinocyte growth factor on tumour growth and small intestinal mucositis after chemotherapy in the rat with breast cancer. Cancer Chemother Pharmacol 50:53–58
Gibson RJ, Keefe DM, Thompson FM, Clarke JM, Goland GJ, Cummins AG (2002) Effect of interleukin-11 on ameliorating intestinal damage after methotrexate treatment of breast cancer in rats. Dig Dis Sci 47:2751–2757
Gibson RJ, Bowen JM, Inglis MR, Cummins AG, Keefe DM (2003) Irinotecan causes severe small intestinal damage, as well as colonic damage, in the rat with implanted breast cancer. J Gastroenterol Hepatol 18:1095–1100
Gibson RJ, Bowen JM, Keefe DM (2005) Palifermin reduces diarrhea and increases survival following irinotecan treatment in tumor-bearing DA rats. Int J Cancer 116:464–470
Govindarajan R (2002) Irinotecan/thalidomide in metastatic colorectal cancer. Oncology (Huntington) 16:23–26
Govindarajan R, Heaton KM, Broadwater R, Zeitlin A, Lang NP, Hauer-Jensen M (2000) Effect of thalidomide on gastrointestinal toxic effects of irinotecan. Lancet 356:566–567
Green D, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1312
Greider C, Chattopadhyay A, Parkhurst C, Yang E (2002) BCL-x(L) and BCL2 delay Myc-induced cell cycle entry through elevation of p27 and inhibition of G1 cyclin-dependent kinases. Oncogene 21:7765–7775
Gross A, McDonnell JM, Korsmeyer SJ (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13:1899–1911
Grossmann J, Artinger M, Grasso AW, Kung HJ, Scholmerich J, Fiocchi C, Levine AD (2001) Hierarchical cleavage of focal adhesion kinase by caspases alters signal transduction during apoptosis of intestinal epithelial cells (comment). Gastroenterology 120:79–88
Haldar S, Jena N, Croce CM (1995) Inactivation of Bcl-2 by phosphorylation. Proc Natl Acad Sci U S A 92:4507–4511
Hall PA, Coates PJ, Ansari B, Hopwood D (1994) Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci 107:3569–3577
Hanada M, Aime-Sempe C, Sato T, Reed JC (1995) Structure–function analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J Biol Chem 270:11962–11969
Hannun YA (1997) Apoptosis and the dilemma of cancer chemotherapy. Blood 89:1845–1853
Hengartner MO (1999) Programmed cell death in the nematode C. elegans. Ann N Y Acad Sci 887:92–104
Hengartner MO, Horvitz HR (1994) The ins and outs of programmed cell death during C. elegans development. Philos Trans R Soc Lond B Biol Sci 345:243–246
Henry-Mowatt J, Dive C, Martinou JC, James D (2004) Role of mitochondrial membrane permeabilization in apoptosis and cancer. Oncogene 23:2850–2860
Herr I, Debatin KM (2001) Cellular stress response and apoptosis in cancer therapy. Blood 98:2603–2614
Herr I, Wilhelm D, Bohler T, Angel P, Debatin KM (1997) Activation of CD95 (APO-1/Fas) signaling by ceramide mediates cancer therapy-induced apoptosis. EMBO J 16:6200–6208
Hershko T, Ginsberg D (2004) Up-regulation of Bcl-2 homology 3 (BH3)-only proteins by E2F1 mediates apoptosis. J Biol Chem 279:8627–8634
Hickman JA, Potten CS, Merritt AJ, Fisher TC (1994). Apoptosis and cancer chemotherapy. Philos Trans R Soc Lond B Biol Sci 345:319–325
Hinds P, Finlay C, Levine AJ (1989) Mutation is required to activate the p53 gene for cooperation with the ras oncogene and transformation. J Virol 63:739–746
Hockenbery DM, Oltvai ZN, Yin XM, Milliman CM, Korsmeyer SJ (1993) Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 75:241–251
Horie T, Matsumoto H, Kasagi M, Sugiyama A, Kikuchi M, Karasawa C, Awazu S, Itakura Y, Fuwa T (1999) Protective effect of aged garlic extract on the small intestinal damage of rats induced by methotrexate treatment. Planta Med 65:545–548
Howarth G, Francis GL, Cool JC, Xu X, Byard RW, Read LC (1996) Milk growth factors enriched from cheese whey ameliorate intestinal damage by methotrexate when administered orally to rats. J Nutr 126:2519–2530
Howarth GS, Shoubridge CA (2001) Enhancement of intestinal growth and repair by growth factors. Curr Opin Pharmacol 1:568–574
Hsu YT, Wolter KG, Youle RJ (1997) Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci U S A 94:3668–3672
Hu ZB, Minden MD, McCulloch EA (1996) Regulation of the synthesis of bcl-2 protein by growth factors. Leukemia 10:1925–1929
Huennekens FM (1994) The methotrexate story: a paradigm for development of cancer chemotherapeutic agents. Adv Enzyme Regul 34:397–419
Ijiri K, Potten CS (1983) Response of intestinal cells of differing topographical and hierarchical status to ten cytotoxic drugs and five sources of radiation. Br J Cancer 47:175–185
Ijiri K, Potten CS (1987) Further studies on the response of intestinal crypt cells of different hierarchical status to eighteen different cytotoxic agents. Br J Cancer 55:113–123
Ikuno N, Soda H, Watanabe M, Oka M (1995) Irinotecan (CPT-11) and characteristic mucosal changes in the mouse ileum and cecum. J Natl Cancer Inst 87:1876–1883
Inomata A, Horii I, Suzuki K (2002) 5-Fluorouracil-induced intestinal toxicity: what determines the severity of damage to murine intestinal crypt epithelia? Toxicol Lett 133:231–240
Jones BA, Gores GJ (1997) Physiology and pathophysiology of apoptosis in epithelial cells of the liver, pancreas, and intestine. Am J Physiol 273:G1174–G1188
Junqueira L, Carnerio J, Long J (eds) (1986) Basic histology. Lange, Los Altos, pp 327–353
Kannan K, Amariglio N, Rechavi G, Jakob-Hirsch J, Kela I, Kaminski N, Getz G, Domany E, Givol D (2001) DNA microarrays identification of primary and secondary target genes regulated by p53. Oncogene 20:2225–2234
Keefe DM (1998) The effect of cytotoxic chemotherapy on the mucosa of the small intestine. M.D. thesis, University of Adelaide, Adelaide
Keefe DM (2004) Gastrointestinal mucositis: a new biological model. Support Care Cancer 12:6–9
Keefe DM, Cummins AG, Dale BM, Kotasek D, Robb TA, Sage RE (1997) Effect of high-dose chemotherapy on intestinal permeability in humans. Clin Sci 92:385–389
Keefe DM, Brealey J, Goland GJ, Cummins AG (2000) Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut 47:632–637
Keefe DM, Gibson RJ, Hauer-Jensen M (2004) Gastrointestinal mucositis. Semin Oncol Nurs 20:38–47
Kelekar A, Thompson CB (1998) Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol 8:324–330
Kelekar A, Chang BS, Harlan JE, Fesik SW, Thompson CB (1997) Bad is a BH3 domain-containing protein that forms an inactivating dimer with Bcl-XL. Mol Cell Biol 17:7040–7046
Kerr JF (1971) Shrinkage necrosis: a distinct mode of cellular death. J Pathol 105:13–20
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Kerr JF, Winterford CM, Harmon BV (1994) Apoptosis. Its significance in cancer and cancer therapy [erratum appears in Cancer 1994 Jun 15; 73(12):3108]. Cancer 73:2013–2026
Khaled AR, Kim K, Hofmeister R, Muegge K, Durum SK (1999) Withdrawal of IL-7 induces Bax translocation from cytosol to mitochondria through a rise in intracellular pH. Proc Natl Acad Sci U S A 96:14476–14481
Kitada S, Krajewski S, Miyashita T, Krajewska M, Reed JC (1996) Gamma-radiation induces upregulation of Bax protein and apoptosis in radiosensitive cells in vivo. Oncogene 12:187–192
Kitada S, Krajewska M, Zhang X, Scudiero D, Zapata JM, Wang HG, Shabaik A, Tudor G, Krajewski S, Myers TG, Johnson GS, Sausville EA, Reed JC (1998) Expression and localization of pro-apoptotic Bcl-2 family protein bad in normal human tissue and tumor cell lines. Am J Pathol 152:51–61
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD (1997) The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis (comment). Science 275:1132–1136
Ko LJ, Prives C (1996) p53: puzzle and paradigm. Genes Dev 10:1054–1072
Komarov PG (1999) A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 285:1733–1737
Komarova EA, Gudkov AV (1998) Could p53 be a target for therapeutic suppression? Semin Cancer Biol 8:389–400
Komarova EA, Gudkov AV (2000) Suppression of p53: a new approach to overcome side effects of antitumor therapy. Biochemistry (Moscow) 65:41–48
Komarova EA, Gudkov AV (2001) Chemoprotection from p53-dependent apoptosis: potential clinical applications of the p53 inhibitors. Biochem Pharmacol 62:657–667
Komarova EA, Neznanov N, Komarov PG, Chernov MV, Wang K, Gudkov AV (2003) p53 inhibitor pifithrin alpha can suppress heat shock and glucocorticoid signaling pathways. J Biol Chem 278:15465–15468
Komarova EA, Kondratov RV, Wang K, Christov K, Golovkina TV, Goldblum JR, Gudkov AV (2004) Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastro-intestinal syndrome in mice. Oncogene 23:3265–3271
Konopleva M, Zhao S, Xie Z, Segall H, Younes A, Claxton DF, Estrov Z, Kornblau SM, Andreeff M (1999) Apoptosis. Molecules and mechanisms. Adv Exp Med Biol 457:217–236
Krajewski S, Krajewska M, Shabaik A, Miyashita T, Wang HG, Reed JC (1994) Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am J Pathol 145:1323–1336
Krajewski S, Krajewska M, Shabaik A, Wang HG, Irie S, Fong L, Reed JC (1994) Immunohistochemical analysis of in vivo patterns of Bcl-X expression. Cancer Res 54:5501–5507
Krajewski S, Bodrug S, Krajewska M, Shabaik A, Gascoyne R, Berean K, Reed JC (1995) Immunohistochemical analysis of Mcl-1 protein in human tissues. Differential regulation of Mcl-1 and Bcl-2 protein production suggests a unique role for Mcl-1 in control of programmed cell death in vivo. Am J Pathol 146:1309–1319
Krajewski S, Krajewska M, Reed JC (1996) Immunohistochemical analysis of in vivo patterns of Bak expression, a proapoptotic member of the Bcl-2 protein family. Cancer Res 56:2849–2855
Krajewska M, Zapata JM, Meinhold-Heerlein I, Hedayat H, Monks A, Handorf H, Shabaik A, Bebendorf L, Lallioniemi O, Kim H, Heifenberger L, Reed JC, Krajewski S (2002) Expression of Bcl-2 family member Bid in normal and malignant tissues. Neoplasia 4:129–140
Lane DP, Crawford LV (1979) T antigen is bound to a host protein in SV40-transformed cells. Nature 278:261–263
Lane DP, Lu X, Hupp T, Hall PA (1994) The role of the p53 protein in the apoptotic response. Philos Trans R Soc Lond B Biol Sci 345:277–280
Leu JI, Dumont P, Hafey M, Murphy ME, George DL (2004) Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol 6:443–450
Leung LK, Wang TT (1999) Differential effects of chemotherapeutic agents on the Bcl-2/Bax apoptosis pathway in human breast cancer cell line MCF-7. Breast Cancer Res Treat 55:73–83
Linette GP, Li Y, Roth K, Korsmeyer SJ (1996) Cross talk between cell death and cell cycle progression: BCL-2 regulates NFAT-mediated activation. Proc Natl Acad Sci U S A 93:9545–9552
Linseman DA, Phelps RA, Bouchard RJ, Le SS, Laessig TA, McClure ML, Heidenreich KA (2002) Insulin-like growth factor-I blocks Bcl-2 interacting mediator of cell death (Bim) induction and intrinsic death signaling in cerebellar granule neurons. J Neurosci 22:9287–9297
Linzer DI, Levine AJ (1979) Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell 17:43–52
Liu QA, Hengartner MO (1999) The molecular mechanism of programmed cell death in C. elegans. Ann N Y Acad Sci 887:92–104
Luo X, Budihardjo I, Zou H, Slaughter C, Wang X (1998) Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481–490
Marshman E, Ottewell PD, Potten CS, Watson AJ (2001) Caspase activation during spontaneous and radiation-induced apoptosis in the murine intestine. J Pathol 195:285–292
Martinon F, Tschopp J (2004) Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell 117:561–574
Marzo I, Brenner C, Zamzami N, Susin SA, Beutner G, Brdiczka D, Remy R, Xie ZH, Reed JC, Kroemer G (1998) The permeability transition pore complex: a target for apoptosis regulation by caspases and bcl-2-related proteins. J Exp Med 187:1261–1271
McDonnell TJ, Beham A, Sarkiss M, Andersen MM, Lo P (1996) Importance of the Bcl-2 family in cell death regulation. Experientia 52:1008–1017
Merritt AJ, Potten CS, Kemp CJ, Hickman JA, Balmain A, Lane DP, Hall PA (1994) The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res 54:614–617
Merritt AJ, Potten CS, Watson AJ, Loh DY, Nakayama K, Hickman JA (1995) Differential expression of bcl-2 in intestinal epithelia. Correlation with attenuation of apoptosis in colonic crypts and the incidence of colonic neoplasia. J Cell Sci 108:2261–2271
Mitchell EP, Schein PS (1984) Gastrointestinal toxicity of chemotherapeutic agents. In: Perry MC, Yarbro JW (eds) Toxicity of chemotherapy. Grune and Stratton, Orlando, pp 269–289
Miyashita T, Reed JC (1993) Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood 81:151–157
Miyashita T, Reed JC (1995) Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80:293–299
Miyashita T, Harigai M, Hanada M, Reed JC (1994) Identification of a p53-dependent negative response element in the bcl-2 gene. Cancer Res 54:3131–3135
Morelli D, Menard S, Colnaghi MI, Balsari A (1996) Oral administration of anti-doxorubicin monoclonal antibody prevents chemotherapy-induced gastrointestinal toxicity in mice. Cancer Res 56:2082–2085
Moss SF, Agarwal B, Arber N, Buan RJ, Krajewska M, Krajewski S, Reed JC, Holt PR (1996) Increased intestinal Bak expression results in apoptosis. Biochem Biophys Res Commun 223:199–203
Muller M, Scaffidi CA, Galle PA, Stremmel W, Krammer PH (1998) The role of p53 and the CD95 (APO-1/Fas) death system in chemotherapy-induced apoptosis. Eur Cytokine Netw 9:685–686
Muller M, Wilder S, Bannasch D, Israeli D, Lehlbach K, Li-Weber M, Friedman SL, Galle PR, Stremmel W, Oren M, Krammer PH (1998) p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J Exp Med 188:2033–2045
Nagai Y, Horie T, Awazu S (1993) Vitamin A, a useful biochemical modulator capable of preventing intestinal damage during methotrexate treatment. Pharmacol Toxicol 73:69–74
Nagata S (1994) Apoptosis regulated by a death factor and its receptor: Fas ligand and Fas. Philos Trans R Soc Lond B Biol Sci 345:281–287
Nakano K, Vousden KH (2001) PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 7:683–694
Narita M, Shimizu S, Ito T, Chittenden T, Lutz RJ, Matsuda H, Tsujimoto Y (1998) Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc Natl Acad Sci U S A 95:14681–14686
Nicholson DW, Thornberry NA (1997) Caspases: killer proteases. Trends Biochem Sci 22:299–306
Nita ME, Nagawa H, Tominaga O, Tsuno N, Fujii S, Sasaki S, Fu CG, Takenoue T, Tsuruo T, Muto T (1998) 5-Fluorouracil induces apoptosis in human colon cancer cell lines with modulation of Bcl-2 family proteins. Br J Cancer 78:986–992
Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N (2000) Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288:1053–1058
Okuno S, Shimizu S, Ito T, Nomura M, Hamada E, Tsujimoto Y, Matsuda H (1998) Bcl-2 prevents caspase-independent cell death. J Biol Chem 273:34272–34277
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619
O’Reilly LA, Cullen L, Visvader J, Lindeman GJ, Print C, Bath ML, Huang DC, Strasser A (2000) The proapoptotic BH3-only protein bim is expressed in hematopoietic, epithelial, neuronal, and germ cells. Am J Pathol 157:449–461
O’Reilly LA, Print C, Hausmann G, Moriishi K, Cory S, Huang DC, Strasser A (2001) Tissue expression and subcellular localization of the pro-survival molecule Bcl-w. Cell Death Differ 8:486–494
Papaconstantinou HT, Chung DH, Zhang W, Ansari NH, Hellmich MR, Townsend CM, Ko TC (2000) Prevention of mucosal atrophy: role of glutamine and caspases in apoptosis in intestinal epithelial cells. J Gastrointest Surg 4:416–423
Papaconstantinou HT, Xie C, Zhang W, Ansari NH, Hellmich MR, Townsend CM, Ko TC (2001) The role of caspases in methotrexate-induced gastrointestinal toxicity. Surgery 130:859–865
Park J, Hockenbery DM (1996) BCL-2, a novel regulator of apoptosis. J Cell Biochem 60:12–17
Pico J, Avila-Garavito A, Naccache P (1998) Mucositis: its occurrence, consequences and treatment in the oncology setting. Oncologist 3:446–451
Pinkoski MJ, Brunner T, Green DR, Lin T (2000) Fas and Fas ligand in gut and liver. Am J Physiol Gastrointest Liver Physiol 278:G354–G366
Potten CS (1990) A comprehensive study of the radiobiological response of the murine (BDF1) small intestine. Int J Radiat Biol 58:925–973
Potten CS (1992) The significance of spontaneous and induced apoptosis in the gastrointestinal tract of mice. Cancer Metastasis Rev 11:179–195
Potten CS (1997) Epithelial cell growth and differentiation. II. Intestinal apoptosis. Am J Physiol 273:G253–G257
Potten CS (1998) Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos Trans R Soc Lond B Biol Sci 353:821–830
Potten CS, Grant HK (1998) The relationship between ionizing radiation-induced apoptosis and stem cells in the small and large intestine. Br J Cancer 78:993–1003
Potten CS, Loeffler M (1990) Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 110:1001–1020
Potten CS, Owen G, Hewitt D, Chadwick CA, Hendry H, Lord BI, Woolford LB (1995) Stimulation and inhibition of proliferation in the small intestinal crypts of the mouse after in vivo administration of growth factors. Gut 36:864–873
Potten CS, Booth C, Pritchard DM (1997) The intestinal epithelial stem cell: the mucosal governor. Int J Exp Pathol 78:219–243
Potten CS, Wilson JW, Booth C (1997) Regulation and significance of apoptosis in the stem cells of the gastrointestinal epithelium. Stem Cells 15:82–93
Pratesi G, Perego P, Zunino F (2001) Role of Bcl-2 and its post-transcriptional modification in response to antitumor therapy. Biochem Pharmacol 61:381–386
Pritchard DM, Potten CS, Hickman JA (1998) The relationships between p53-dependent apoptosis, inhibition of proliferation, and 5-fluorouracil-induced histopathology in murine small intestinal epithelia. Cancer Res 58:5453–5465
Pritchard DM, Potten CS, Korsmeyer SJ, Roberts S, Hickman JA (1999) Damage-induced apoptosis in intestinal epithelia from bcl-2-null and bax-null mice: investigations of the mechanistic determinants of epithelial apoptosis in vivo. Oncogene 18:7287–7293
Pritchard DM, Print C, O’Reilly L, Adams JM, Potten CS, Hickman JA (2000) Bcl-w is an important determinant of damage-induced apoptosis in epithelia of small and large intestine. Oncogene 19:3955–3959
Puthalakath DC, Huang LA, O’Reilly SM, King A, Strasser A (1999) The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell 3:287–296
Puthalakath H, Strasser A (2002) Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ 9:505–512
Raisova M, Hossini AM, Eberle J, Riebeling C, Wieder T, Sturm I, Daniel PT, Orfanos PE, Geilen CC (2001) The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Invest Dermatol 117:333–340
Reed JC (2000) Mechanisms of apoptosis. Am J Pathol 157:1415–1430
Renehan A, Gossiel R, Davidson SE, Roberts SA, Chadwick C, Wilks DP, Potten CS, Hendry JH, Hunter RD, Renehan AG (1995) What is apoptosis, and why is it important? Radiother Oncol 37:1–9
Renehan AG, Bach SP, Potten CS (2001) The relevance of apoptosis for cellular homeostasis and tumorigenesis in the intestine. Can J Gastroenterol 15:166–176
Rosse T (1998) Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature 391:486–499
Sabbatini M, Bozzo C, Castellucci M, Cannas M (2004) Morphometric quantification of apoptotic stages in cell culture. Cells Tissues Organs 178:139–145
Sax JK, Fei P, Murphy ME, Bernhard E, Korsmeyer SJ, El-Deiry WS (2002) BID regulation by p53 contributes to chemosensitivity. Nat Cell Biol 4:842–849
Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME (1998) Two CD95 (APO-1/Fas) signaling pathways. EMBO J 17:1675–1687
Schafer T, Scheuer C, Roemer K, Menger MD, Vollmar B (2003) Inhibition of p53 protects liver tissue against endotoxin-induced apoptotic and necrotic cell death. FASEB J 17:660–667
Schinzel A, Kaufmann T, Borner C (2004) Bcl-2 family members: intracellular targeting, membrane-insertion, and changes in subcellular localization. Biochim Biophys Acta 1644:95–105
Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB, Korsmeyer SJ (1995) Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci U S A 92:7834–7838
Sherwood L (1997) Human physiology—from cells to systems, 3rd edn. Wadsworth, Belmont, pp 446–600
Shibue T, Takeda K, Oda E, Tanaka H, Murasawa H, Takaoka A, Morishita Y, Akira S, Taniguchi T, Tanaka N (2003) Integral role of Noxa in p53-mediated apoptotic response. Genes Dev 17:2233–2238
Shimizu S, Konishi A, Kodama T, Tsujimoto Y (2000) BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. [erratum appears in Proc Natl Acad Sci U S A 2000 Aug 1; 97(16):9347]. Proc Natl Acad Sci U S A 97:3100–3105
Shinohara H, Killion JJ, Kuniyasu H, Kumar R, Fidler IJ (1998) Prevention of intestinal toxic effects and intensification of irinotecan’s therapeutic efficacy against murine colon cancer liver metastases by oral administration of the lipopeptide JBT 3002. Clin Cancer Res 4:2053–2063
Slee EA, Adrain C, Martin SJ (1999) Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ 6:1067–1074
Slee EA, O’Connor DJ, Lu X (2004) To die or not to die: how does p53 decide? Oncogene 23:2809–2818
Smith ND, Rubenstein JN, Eggener SE, Kozlowski JM (2003) The p53 tumor suppressor gene and nuclear protein: basic science review and relevance in the management of bladder cancer. J Urol 169:1219–1228
Solary E, Favre B, Caillot D, Sidaner I, Guy H (2000) Positive and negative regulation of apoptotic pathways by cytotoxic agents in hematological malignancies. Leukemia 14:1833–1849
Sonis ST (2004) The pathobiology of mucositis. Nat Rev Cancer 4:277–284
Sonis ST (2004) Pathobiology of mucositis. Semin Oncol Nurs 20:11–15
Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, Bekele BN, Raber-Durlacher J, Donnelly JP, Rubenstein EB (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100:1995–2025
Srivastava RK, Srivastava AR, Korsmeyer SJ, Nesterova M, Cho-Chung YS, Longo DL (1998) Involvement of microtubules in the regulation of Bcl2 phosphorylation and apoptosis through cyclic AMP-dependent protein kinase. Mol Cell Biol 18:3509–3517
Strasser A, O’Connor L, Dixit VM (2000) Apoptosis signaling. Ann Rev Biochem 69:217–245
Strasser A, Puthalakath H, Bouillet P, Huang DC, O’Connor L, O’Reilly LA, Cullen L, Cory S, Adams JM (2000) The role of bim, a proapoptotic BH3-only member of the Bcl-2 family in cell-death control. Ann N Y Acad Sci 917:541–548
Susin SA, Zamzami N, Kroemer G (1998) Mitochondria as regulators of apoptosis: doubt no more. Biochim Biophys Acta 1366:151–165
Takasuna K, Hagiwara T, Hirohashi M, Kato M, Nomura M, Nagai E, Yokoi T, Kamataki T (1996) Involvement of beta-glucuronidase in intestinal microflora in the intestinal toxicity of the antitumor camptothecin derivative irinotecan hydrochloride (CPT-11) in rats. Cancer Res 56:3752–3757
Tamatani M, Ogawa S, Nunez G, Tohyama M (1998) Growth factors prevent changes in Bcl-2 and Bax expression and neuronal apoptosis induced by nitric oxide. Cell Death Differ 5:911–919
Taminiau JA, Gall DG, Hamilton JR (1980) Response of the rat small-intestine epithelium to methotrexate. Gut 21:486–492
Tarnawski AS, Szabo I (2001) Apoptosis—programmed cell death and its relavence to gastrointestinal epithelium: survival signal from the matrix. Gastroenterology 120:294–299
Tenenbaun L (1994) Cancer chemotherapy and biotherapy. Saunders, Philadephia, pp 7–8
Thornberry NA (1998) Caspases: key mediators of apoptosis. Chem Biol 5:R97–R103
Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281:1312–1316
Tran CD, Howarth GS, Coyle P, Philcox JC, Rofe AM, Butler RN (2003) Dietary supplementation with zinc and a growth factor extract derived from bovine cheese whey improves methotrexate-damaged rat intestine. Am J Clin Nutr 77:1296–1303
Trier JS (1962) Morphologic alterations induced by methotrexate in the mucosa of human proximal intestine. I. Serial observations by light microscopy. Gastroenterology 42:295–305
van Loo G, Saelens X, van Gurp M, MacFarlane M, Martin SJ, Vandenabeele P (2002) The role of mitochondrial factors in apoptosis: a Russian roulette with more than one bullet. Cell Death Differ 9:1031–1042
van’t Land B, Meijer HP, Frerichs J, Koetsier M, Jager D, Smeets RL, M’Rabet L, Hoijer M (2002) Transforming growth factor-beta2 protects the small intestine during methotrexate treatment in rats possibly by reducing stem cell cycling. Br J Cancer 87:113–118
van’t Land B, van Beek NM, van den Berg JJ, M’Rabet L (2004) Lactoferrin reduces methotrexate-induced small intestinal damage, possibly through inhibition of GLP-2-mediated epithelial cell proliferation. Dig Dis Sci 49:425–433
Verburg M, Renes IB, Meijer HP, Taminiau HP, Buller HA, Einerhand AW, Dekker J (2000) Selective sparing of goblet cells and paneth cells in the intestine of methotrexate-treated rats. Am J Physiol Gastrointest Liver Physiol 279:G1037–G1047
Vogelstein B, Lane D, Levine AJ (2000) Surfing the p53 network. Nature 408:307–310
Vollmar B, El-Gibaly AM, Scheuer C, Strik MW, Bruch HP, Menger HP (2002) Acceleration of cutaneous wound healing by transient p53 inhibition. Lab Invest 82:1063–1071
Vousden KH, Lu X (2002) Live or let die: the cell’s response to p53. Nat Rev Cancer 2:594–604
Wang JY, Naderi S, Chen TT (2001) Role of retinoblastoma tumor suppressor protein in DNA damage response. Acta Oncol 40:689–695
Watson AJ (1995) Necrosis and apoptosis in the gastrointestinal tract. Aliment Pharmacol Ther 9:215–226
Wei MC (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727–730
Weller M (1998) Predicting response to cancer chemotherapy: the role of p53. Cell Tissue Res 292:435–445
Westcarr S, Farshori P, Wyche J, Anderson WA (1999) Apoptosis and differentiation in the crypt-villus unit of the rat small intestine. J Submicrosc Cyto Pathol 31:15–30
Wilson JW, Pritchard DM, Hickman JA, Potten CS (1998) Radiation-induced p53 and p21WAF-1/CIP1 expression in the murine intestinal epithelium: apoptosis and cell cycle arrest. Am J Pathol 153:899–909
Yang E, Korsmeyer SJ (1996) Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood 88:386–401
Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ (1995) Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell 80:285–291
Yin XM, Oltvai ZN, Veis-Novack DJ, Linette GP (1995) Bcl-2 gene family and the regulation of programmed cell death. Biochim Biophys Acta 1271:63–66
Zhang M, Liu W, Ding D, Salvi R (2003) Pifithrin-α supresses p53 and protects cochlear and vestibular hair cells from cisplatin-induced apoptosis. Neuroscience 120:191–205
Zhu M, Yu QS, Cutler RG, Culmsee CW, Holloway HW, Lahiri DK, Mattson MP, Greig NH (2002) Novel p53 inactivators with neuroprotective action: syntheses and pharmacological evaluation of 2-imino-2,3,4,5,6,7-hexahydrobenzothiazole and 2-imino-2,3,4,5,6,7-hexahydrobenzoxazole derivatives. J Med Chem 45:5090–5097
Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB (2001) BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev 15:1481–1486
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bowen, J.M., Gibson, R.J., Cummins, A.G. et al. Intestinal mucositis: the role of the Bcl-2 family, p53 and caspases in chemotherapy-induced damage. Support Care Cancer 14, 713–731 (2006). https://doi.org/10.1007/s00520-005-0004-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-005-0004-7