Abstract
Purpose
Intestinal mucositis and the closely associated diarrhea are common costly side effects of irinotecan. Cytokine modulators, such as thalidomide and pentoxifylline, are found capable of attenuating intestinal mucositis progression. Nitric oxide (NO) seems to be a key mediator of the antineoplastic drug toxicity. The aim of this study was to investigate the role of NO on the pathogenesis of intestinal mucositis, as well as the participation of cytokines upon inducible nitric oxide synthase (iNOS) expression in irinotecan-induced intestinal mucositis.
Methods
iNOS-knockout (iNOS−/−) and C57BL/6 (WT, wild type) animals (n = 5–6) were given either saline or irinotecan (60 mg/kg i.p for 4 days), with or without pretreatment with aminoguanidine (50 mg/kg s.c.), thalidomide (60 mg/kg s.c), infliximab (5 mg/kg i.v.), or pentoxifylline (1.7 mg/kg s.c). On day 5, diarrhea was assessed, and following euthanasia, proximal intestinal samples were obtained for myeloperoxidase (MPO) and iNOS activity, morphometric analysis, western blot and immunohistochemistry to iNOS, cytokine dosage, and for in vitro evaluation of gut contractility.
Results
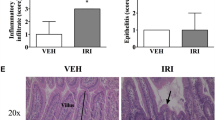
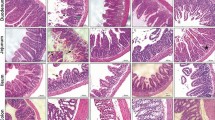
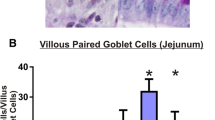
Irinotecan induced severe diarrhea and intestinal smooth muscle over-contractility, accompanied with histopathological changes. Additionally, increased MPO and iNOS activity and iNOS immunoexpression were found in WT animals treated with irinotecan. The rise in MPO, smooth muscle over-contractility, and diarrhea were abrogated in aminoguanidine-treated and iNOS−/− mice. Moreover, through western blot, we verified that infliximab and pentoxifylline significantly inhibited irinotecan-induced iNOS expression. In addition, cytokine concentration was found only partially decreased in irinotecan-treated iNOS−/− mice when compared with wild-type animals that were given irinotecan.
Conclusions
This study suggests a role of nitric oxide in the pathogenesis of irinotecan-induced intestinal mucositis and also provides evidence for the participation of cytokines on iNOS induction.






Similar content being viewed by others
References
Rubenstein EB, Peterson DE, Schubert M, Keefe D, McGuire D, Epstein J et al (2004) Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 100:2026–2046
Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE et al (2007) Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 109:820–831
Blijlevens N, Sonis S (2007) Palifermin (recombinant keratinocyte growth factor-1): a pleiotropic growth factor with multiple biological activities in preventing hemotherapy- and radiotherapy-induced mucositis. Ann Oncol 18:817–826
Chester JD, Joel SP, Cheeseman SL, Hall GD, Braun MS, Perry J et al (2003) Phase I and pharmacokinetic study of intravenous irinotecan plus oral ciclosporin in patients with fluorouracil-refractory metastatic colon cancer. J Clin Oncol 21:1125–1132
Rocha-Lima CM, Green MR, Rotche R, Miller WH Jr, Jeffrey GM, Cisar LA et al (2004) Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol 22:18–21
Gibson RJ, Keefe DM (2006) Cancer chemotherapy-induced diarrhoea and constipation: mechanisms of damage and prevention strategies. Support Care Cancer 14:890–900
Melo ML, Brito GA, Soares RC, Carvalho SB, Silva JV, Soares PM et al (2008) Role of cytokines (TNF-alpha, IL-1beta and KC) in the pathogenesis of CPT-11-induced intestinal mucositis in mice: effect of pentoxifylline and thalidomide. Cancer Chemother Pharmacol 61:775–784
Yip KH, Huang Y, Waye MM, Lau HY (2008) Induction of nitric oxide synthases in primary human cultured mast cells by IgE and proinflammatory cytokines. Int Immunopharmacol 8:764–768
Nathan C (1997) Inducible nitric oxide synthase: what difference does it make? J Clin Invest 100:2417–2423
Rios-Santos F, Alves-Filho JC, Souto FO, Spiller F, Freitas A, Lotufo CMC et al (2007) Dow-regulation of CXCR2 on neutrophils in severe sepsis is mediated by inducible nitric oxide synthase-derived nitric oxide. Am J Respir Crit Care Med 175:490–497
Gómez-Cambronero L, Camps B, de La Asunción JG, Cerdá M, Pellín A, Pallardó FV et al (2000) Pentoxifylline ameliorates cerulein-induced pancreatitis in rats: role of glutathione and nitric oxide. J Pharmacol Exp Ther 293:670–676
Ribeiro RA, Freitas HC, Campos MC, Santos CC, Figueiredo FC, Brito GA, Cunha FQ (2002) Tumor necrosis factor-alpha and interleukin-1beta mediate the production of nitric oxide involved in the pathogenesis of ifosfamide induced hemorrhagic cystitis in mice. J Urol 167:2229–2234
Leitão RF, Ribeiro RA, Bellaguarda EA, Macedo FD, Silva LR, Oriá RB et al (2007) Role of nitric oxide on pathogenesis of 5-fluorouracil induced experimental oral mucositis in hamster. Cancer Chemother Pharmacol 59:603–612
Ikuno N, Soda H, Watanabe M, Oka M (1995) Irinotecan (CPT-11) and characteristic mucosal changes in the mouse ileum and caecum. J Natl Cancer Inst 87:1876–1883
Bradley PP, Christensen RD, Rothstein G (1982) Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood 60:618–622
Bredt DS, Snyder SH (1989) Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci USA 86:9030–9033
Hsu SM, Raine L (1981) Protein A, avidin, and biotin in immunohistochemistry. J Histochem Cytochem 29:1349–1353
Yeoh AS, Bowen JM, Gibson RJ, Keefe DM (2005) Nuclear factor kappaB (NFkappaB) and cyclooxygenase-2 (Cox-2) expression in the irradiated colorectum is associated with subsequent histopathological changes. Int J Radiat Oncol Biol Phys 63:1295–1303
Kurita A, Kado S, Kaneda N, Onoue M, Hashimoto S, Yokokura T (2000) Modified irinotecan hydrochloride (CPT-11) administration schedule improves induction of delayed-onset diarrhea in rats. Cancer Chemother Pharmacol 46:211–220
Araújo PV, Clemente CM, da Graça JR, Rola FH, de Oliveira RB, dos Santos AA, Magalhães PJ (2005) Inhibitory effect of sildenafil on rat duodenal contractility in vitro: putative cGMP involvement. Clin Exp Pharmacol Physiol 32:191–195
Cunha FQ, Assreuy J, Moss DW, Rees D, Leal LM, Moncada S et al (1994) Differential induction of nitric oxide synthase in various organs of the mouse during endotoxaemia: role of TNF-alpha and IL-1-beta. Immunology 81:211–215
Huie RE, Padmaja S (1993) The reaction of NO with superoxide. Free Radic Res Commun 18:195–199
Beckman JS (1996) Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Toxicol 9:836–844
Messmer UK, Ankarcrona M, Nicotera P et al (1994) p53 expression in nitric oxide-induced apoptosis. FEBS Lett 355:23–26
Nguyen T, Brunson D, Crespi CL et al (1992) DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci USA 89:3030–3034
Forstermann U, Closs EI, Pollock JS et al (1994) Nitric oxide synthase isozymes: characterization, purification, molecular cloning, and functions. Hypertension 23:1121–1131
Kurose I, Ebinuma H, Higuchi H et al (1995) Nitric oxide mediates mitochondrial dysfunction in hepatoma cells induced by nonactivated Kupffer cells: evidence implicating ICAM-1-dependent process. J Gastroenterol Hepatol 10:68–71
Xu DZ, Lu Q, Deitch EA (2002) Nitric oxide directly impairs intestinal barrier function. Shock 17:139–145
Shah V, Lyford G, Gores G, Farrugia G (2004) Nitric oxide in gastrointestinal health and disease. Gastroenterology 126:903–913
Franks ME, Macpherson GR, Figg WD (2004) Thalidomide. Lancet 363:1802–1811
Efthimiou P, Markenson JA (2005) Role of biological agents in immune-mediated inflammatory diseases. South Med J 98:192–204
Lima V, Brito GAC, Cunha FQ, Rebouças CG, Falcão BA, Augusto RF (2005) Effects of the tumour necrosis factor-α inhibitors pentoxifylline and thalidomide in short-term experimental oral mucositis in hamsters. Eur J Oral Sci 113:210–217
Govindarajan R, Heaton KM, Broadwater R, Zeitlin A, Lang NP, Hauer-Jensen M (2000) Effect of thalidomide on gastrointestinal toxic effects of irinotecan. Lancet 356:566–567
Allegrini G, Di Paolo A, Cerri E, Cupini S, Amatori F, Masi G et al (2006) Irinotecan in combination with thalidomide in patients with advanced solid tumors: a clinical study with pharmacodynamic and pharmacokinetic evaluation. Cancer Chemother Pharmacol 58:585–593
Sampaio EP, Sarno EN, Galilly R, Cohn ZA, Kaplan G (1991) Thalidomide selectively inhibits tumor necrosis factor α production by stimulated human monocytes. J Exp Med 173:699–703
Peterson PK, Hu S, Sheng WS et al (1995) Thalidomide inhibits tumor necrosis factor-α production by lipopolysaccharide- and lipoarabinomannan-stimulated human microglial cells. J Infect Dis 172:1137–1140
Makonkawkeyoon S, Limson-Pobre RNR, Moreira AL, Schauf V, Kaplan G (1993) Thalidomide inhibits the replication of human immunodeficiency virus type 1. Proc Natl Acad Sci USA 90:5974–5978
Sampaio EP, Moreira AL, Sarno EN, Malta AM, Kaplan G (1992) Prolonged treatment with recombinant interferon α induces erythema nodosum leprosum in lepromatous leprosy patients. J Exp Med 175:1729–1737
Tramontana JM, Utaipat U, Molloy A et al (1995) Thalidomide treatment reduces tumor necrosis factor α production and enhances weight gain in patients with pulmonary tuberculosis. Mol Med 1:384–397
Badamtseren B, Odkhuu E, Koide N, Haque A, Naiki Y, Hashimoto S, Komatsu T, Yoshida T, Yokochi T (2011) Thalidomide inhibits interferon-γ-mediated nitric oxide production in mouse vascular endothelial cells. Cell Immunol 270(1):19–24
Genovese T, Mazzon E, Esposito E, Di Paola R, Caminiti R, Meli R, Bramanti P, Cuzzocrea S (2008) Effect of thalidomide on signal transduction pathways and secondary damage in experimental spinal cord trauma. Shock 30(3):231–240
Kreth S, Ledderose C, Luchting B, Weis F, Thiel M (2010) Immunomodulatory properties of pentoxifylline are mediated via adenosine-dependent pathways. Shock 34(1):10–16
Beshay E, Croze F, Prud’homme GJ (2001) The phosphodiesterase inhibitors pentoxifylline and rolipram suppress macrophage activation and nitric oxide production in vitro and in vivo. Clin Immunol 98(2):272–279
Wang W, Zolty E, Falk S, Basava V, Reznikov L, Schrier R (2006) Pentoxifylline protects against endotoxin-induced acute renal failure in mice. Am J Physiol Renal Physiol 291(5):F1090–F1095
Bogdan C (1997) Of microbes, macrophages and nitric oxide. Behring Inst Mitt 99:58–72
Bowen JM, Keefe DM (2008) New pathways for alimentary mucositis. J Oncol. 2008:907892. doi:10.1155/2008/907892
Jaeschke H, Williams CD, Ramachandran A, Bajt ML (2011) Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. doi: 10.1111/j.1478-3231.2011.02501.x
Lopez-Ferrer D, Squier TC (2011) Aging enhances production of reactive oxygen species and bactericidal activity in peritoneal macrophages by up-regulating classical activation pathways. Biochemistry. doi:10.1021/bi2011866
Soares PM, Mota JM, Gomes AS, Oliveira RB, Assreuy AM, Brito GA et al (2008) Gastrointestinal dysmotility in 5-fluorouracil-induced intestinal mucositis outlasts inflammatory process resolution. Cancer Chemother Pharmacol 63:91–98
Mihara H, Boudaka A, Shibasaki K, Yamanaka A, Sugiyama T, Tominaga M (2010) Involvement of TRPV2 activation in intestinal movement through nitric oxide production in mice. J Neurosci 30(49):16536–16544
Grasa L, Rebollar E, Arruebo MP, Plaza MA, Murillo MD (2005) The role of NO in the contractility of rabbit small intestine in vitro: effect of K + channels. J Physiol Pharmacol 56:407–419
Caballero-Alomar C, Santos C, Lopez D, Metafile MT, Puig-Parellada P (2003) Sources and implications of basal nitric oxide in spontaneous contractions of guinea pig taenia caeci. Am J Physiol 285:747–753
Kalff JC, Schraut WH, Billiar TR, Simmons RL, Bauer AJ (2000) Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology 118(2):316–327
Tan-No K, Niijima F, Nakagawasai O, Sato T, Satoh S, Tadano T (2003) Development of tolerance to the inhibitory effect of loperamide on gastrointestinal transit in mice. Eur J Pharm Sci 20:357–363
Lundberg S, Holst M, Hellström PM (2006) Expression of iNOS mRNA associated with suppression of colonic contraction in rat colitis. Acta Physiol (Oxf) 187(4):489–494
Acknowledgments
We are grateful to José Ivan Rodrigues, Maria Silvandira Freire, and Antônio Ricardo Barreto for technical assistance. This work was supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), CAPES (Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), and FUNCAP (Fundação Cearense de Apoio ao Desenvolvimento Científico). G.A.C.B., M.H.L.P., and R.A.R. are recipients of Conselho Nacional de Desenvolvimento Científico e Tecnológico fellowships.
Conflict of interest
The authors indicated no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lima-Júnior, R.C.P., Figueiredo, A.A., Freitas, H.C. et al. Involvement of nitric oxide on the pathogenesis of irinotecan-induced intestinal mucositis: role of cytokines on inducible nitric oxide synthase activation. Cancer Chemother Pharmacol 69, 931–942 (2012). https://doi.org/10.1007/s00280-011-1780-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1780-z