Abstract
Rationale
The legalization of medicinal use of Cannabis sativa in most US states and the removal of hemp from the Drug Enforcement Agency (DEA) controlled substances act has resulted in a proliferation of products containing Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) for oral consumption (e.g., edibles, oils, and tinctures) that are being used for recreational and medicinal purposes.
Objective
This study examined the effects of cannabinoids THC and CBD when administered orally on measures of pain sensitivity, body temperature, locomotor activity, and catalepsy (i.e., cannabinoid tetrad) in male and female Sprague Dawley rats.
Methods
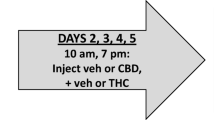
Rats (N = 24, 6 per sex/drug group) were administered THC (1–20 mg/kg), CBD (3–30 mg/kg), or sesame oil via oral gavage. Thermal and mechanical pain sensitivity (tail flick assay, von Frey test), rectal measurements for body temperature, locomotor activity, and the bar-test of catalepsy were completed. A separate group of rats (N = 8/4 per sex) was administered morphine (5–20 mg/kg; intraperitoneal, IP) and evaluated for pain sensitivity as a positive control.
Results
We observed classic tetrad effects of antinociception, hypothermia, hyper- and hypolocomotion, and catalepsy after oral administration of THC that were long lasting (> 7 h). CBD modestly increased mechanical pain sensitivity and produced sex-dependent effects on body temperature and locomotor activity.
Conclusions
Oral THC and CBD produced long lasting effects that differed in magnitude and time course when compared with other routes of administration. Examination of cannabinoid effects administered via different routes of administration, species, and in both males and females is critical to enhance translation.





Similar content being viewed by others
Abbreviations
- THC:
-
Δ9-Tetrahydrocannabinol
- CBD:
-
Cannabidiol
- CB1R:
-
Cannabinoid receptor 1
- IP:
-
Intraperitoneal
- SC:
-
Subcutaneous
- IV:
-
Intravenous
- TF:
-
Tail flick
- MPE:
-
Maximum possible effect
References
Abraham AD, Leung EJY, Wong BA, Rivera ZMG, Kruse LC, Clark JJ, Land BB (2020) Orally consumed cannabinoids provide long-lasting relief of allodynia in a mouse model of chronic neuropathic pain. Neuropsychopharmacology 45(7):1105–1114
Booker L, Naidu PS, Razdan RK, Mahadevan A, Lichtman AH (2009) Evaluation of prevalent phytocannabinoids in the acetic acid model of visceral nociception. Drug Alcohol Depend 105(1–2):42–47
Britch SC, Wiley JL, Yu Z, Clowers BH, Craft RM (2017) Cannabidiol-Delta(9)-tetrahydrocannabinol interactions on acute pain and locomotor activity. Drug Alcohol Depend 175:187–197
Burstein S, Hunter SA, Latham V, Renzulli L (1987) A major metabolite of delta 1-tetrahydrocannabinol reduces its cataleptic effect in mice. Experientia 43(4):402–403
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53(1):55–63
Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, Martin BR (1993) Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther 265(1):218–226
Cooper ZD, Craft RM (2018) Sex-dependent effects of cannabis and cannabinoids: a translational perspective. Neuropsychopharmacology 43(1):34–51
Craft RM, Britch SC, Buzitis NW, Clowers BH (2019) Age-related differences in Delta(9)-tetrahydrocannabinol-induced antinociception in female and male rats. Exp Clin Psychopharmacol 27(4):338–347
Dixon WJ (1991) Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev 15(1):47–50
Dow-Edwards D, Zhao N (2008) Oral THC produces minimal behavioral alterations in preadolescent rats. Neurotoxicol Teratol 30(5):385–389
Farquhar CE, Breivogel CS, Gamage TF, Gay EA, Thomas BF, Craft RM, Wiley JL (2019) Sex, THC, and hormones: effects on density and sensitivity of CB1 cannabinoid receptors in rats. Drug Alcohol Depend 194:20–27
Ferre S, Guix T, Prat G, Jane F, Casas M (1990) Is experimental catalepsy properly measured? Pharmacol Biochem Behav 35(4):753–757
Finn DP, Haroutounian S, Hohmann AG, Krane E, Soliman N, Rice ASC (2021) Cannabinoids, the endocannabinoid system, and pain: a review of preclinical studies. Pain 162(Suppl 1):S5–S25
Gallily R, Yekhtin Z, Hanuš LO (2015) Overcoming the bell-shaped dose-response of cannabidiol by using cannabis extract enriched in cannabidiol. Pharmacology & Pharmacy 6(02):75
Gomes FV, Del Bel EA, Guimaraes FS (2013) Cannabidiol attenuates catalepsy induced by distinct pharmacological mechanisms via 5-HT1A receptor activation in mice. Prog Neuropsychopharmacol Biol Psychiatry 46:43–47
Grotenhermen F (2003) Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 42(4):327–360
Guimaraes FS, Chiaretti TM, Graeff FG, Zuardi AW (1990) Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology 100(4):558–559
Henderson-Redmond AN, Sepulveda DE, Ferguson EL, Kline AM, Piscura MK, Morgan DJ (2021) Sex-specific mechanisms of tolerance for the cannabinoid agonists CP55,940 and delta-9-tetrahydrocannabinol (Delta(9)-THC). Psychopharmacology (Berl)
Hlozek T, Uttl L, Kaderabek L, Balikova M, Lhotkova E, Horsley RR, Novakova P, Sichova K, Stefkova K, Tyls F, Kuchar M, Palenicek T (2017) Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur Neuropsychopharmacol 27(12):1223–1237
Javadi-Paydar M, Nguyen JD, Kerr TM, Grant Y, Vandewater SA, Cole M, Taffe MA (2018) Effects of Delta9-THC and cannabidiol vapor inhalation in male and female rats. Psychopharmacology 235(9):2541–2557
Javadi-Paydar M, Creehan KM, Kerr TM, Taffe MA (2019) Vapor inhalation of cannabidiol (CBD) in rats. Pharmacol Biochem Behav 184:172741
Jesus CHA, Redivo DDB, Gasparin AT, Sotomaior BB, de Carvalho MC, Genaro K, Zuardi AW, Hallak JEC, Crippa JA, Zanoveli JM, da Cunha JM (2019) Cannabidiol attenuates mechanical allodynia in streptozotocin-induced diabetic rats via serotonergic system activation through 5-HT1A receptors. Brain Res 1715:156–164
King KM, Myers AM, Soroka-Monzo AJ, Tuma RF, Tallarida RJ, Walker EA, Ward SJ (2017) Single and combined effects of Delta(9) -tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy-induced neuropathic pain. Br J Pharmacol 174(17):2832–2841
Kruse LC, Cao JK, Viray K, Stella N, Clark JJ (2019) Voluntary oral consumption of Delta(9)-tetrahydrocannabinol by adolescent rats impairs reward-predictive cue behaviors in adulthood. Neuropsychopharmacology 44(8):1406–1414
Linher-Melville K, Zhu YF, Sidhu J, Parzei N, Shahid A, Seesankar G, Ma D, Wang Z, Zacal N, Sharma M, Parihar V, Zacharias R, Singh G (2020) Evaluation of the preclinical analgesic efficacy of naturally derived, orally administered oil forms of Delta9-tetrahydrocannabinol (THC), cannabidiol (CBD), and their 1:1 combination. PLoS One 15(6), e0234176.
Long LE, Chesworth R, Huang XF, McGregor IS, Arnold JC, Karl T (2010) A behavioural comparison of acute and chronic Delta9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. Int J Neuropsychopharmacol 13(7):861–876
Lunn S, Diaz P, O’Hearn S, Cahill SP, Blake A, Narine K, Dyck JRB (2019) Human pharmacokinetic parameters of orally administered Delta(9)-tetrahydrocannabinol capsules are altered by fed versus fasted conditions and sex differences. Cannabis Cannabinoid Res 4(4):255–264
Marshell R, Kearney-Ramos T, Brents LK, Hyatt WS, Tai S, Prather PL, Fantegrossi WE (2014) In vivo effects of synthetic cannabinoids JWH-018 and JWH-073 and phytocannabinoid Delta9-THC in mice: inhalation versus intraperitoneal injection. Pharmacol Biochem Behav 124:40–47
Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ (1991) Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav 40(3):471–478
Marusich JA, Lefever TW, Antonazzo KR, Craft RM, Wiley JL (2014) Evaluation of sex differences in cannabinoid dependence. Drug Alcohol Depend 137:20–28
Metna-Laurent M, Mondesir M, Grel A, Vallee M, Piazza PV (2017) Cannabinoid-induced tetrad in mice. Curr Protoc Neurosci 80, 9 59 51–59 59 10
Mlost J, Bryk M, Starowicz K (2020) Cannabidiol for pain treatment: focus on pharmacology and mechanism of action. Int J Mol Sci 21(22)
Moore CF, Davis CM, Harvey EL, Taffe MA, Weerts EM (2021) Appetitive, antinociceptive, and hypothermic effects of vaped and injected Delta-9-tetrahydrocannabinol (THC) in rats: exposure and dose-effect comparisons by strain and sex. Pharmacol Biochem Behav 202:173116
Nadulski T, Pragst F, Weinberg G, Roser P, Schnelle M, Fronk EM, Stadelmann AM (2005) Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Delta9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Ther Drug Monit 27(6):799–810
Newmeyer MN, Swortwood MJ, Andersson M, Abulseoud OA, Scheidweiler KB, Huestis MA (2017) Cannabis edibles: blood and oral fluid cannabinoid pharmacokinetics and evaluation of oral fluid screening devices for predicting delta(9)-tetrahydrocannabinol in blood and oral fluid following Cannabis Brownie Administration. Clin Chem 63(3):647–662
Nguyen JD, Aarde SM, Vandewater SA, Grant Y, Stouffer DG, Parsons LH, Cole M, Taffe MA (2016) Inhaled delivery of Delta(9)-tetrahydrocannabinol (THC) to rats by e-cigarette vapor technology. Neuropharmacology 109:112–120
Nguyen JD, Grant Y, Kerr TM, Gutierrez A, Cole M, Taffe MA (2018) Tolerance to hypothermic and antinoceptive effects of 9-tetrahydrocannabinol (THC) vapor inhalation in rats. Pharmacol Biochem Behav 172:33–38
Prescott WR, Gold LH, Martin BR (1992) Evidence for separate neuronal mechanisms for the discriminative stimulus and catalepsy induced by delta 9-THC in the rat. Psychopharmacology 107(1):117–124
Puighermanal E, Busquets-Garcia A, Gomis-Gonzalez M, Marsicano G, Maldonado R, Ozaita A (2013) Dissociation of the pharmacological effects of THC by mTOR blockade. Neuropsychopharmacology 38(7):1334–1343
Rock EM, Connolly C, Limebeer CL, Parker LA (2016) Effect of combined oral doses of Delta(9)-tetrahydrocannabinol (THC) and cannabidiolic acid (CBDA) on acute and anticipatory nausea in rat models. Psychopharmacology 233(18):3353–3360
Rohleder C, Pahlisch F, Graf R, Endepols H, Leweke FM (2020) Different pharmaceutical preparations of Delta(9) -tetrahydrocannabinol differentially affect its behavioral effects in rats. Addict Biol 25(3), e12745
Sanberg PR, Bunsey MD, Giordano M, Norman AB (1988) The catalepsy test: its ups and downs. Behav Neurosci 102(5):748–759
Sanudo-Pena MC, Romero J, Seale GE, Fernandez-Ruiz JJ, Walker JM (2000) Activational role of cannabinoids on movement. Eur J Pharmacol 391(3):269–274
Sofia RD, Vassar HB, Knobloch LC (1975) Comparative analgesic activity of various naturally occurring cannabinoids in mice and rats. Psychopharmacologia 40(4):285–295
Spindle TR, Bonn-Miller MO, Vandrey R (2019) Changing landscape of cannabis: novel products, formulations, and methods of administration. Curr Opin Psychol 30:98–102
Taffe MA, Creehan KM, Vandewater SA (2015) Cannabidiol fails to reverse hypothermia or locomotor suppression induced by Delta(9) -tetrahydrocannabinol in Sprague-Dawley rats. Br J Pharmacol 172(7):1783–1791
Tai S, Hyatt WS, Gu C, Franks LN, Vasiljevik T, Brents LK, Prather PL, Fantegrossi WE (2015) Repeated administration of phytocannabinoid Delta(9)-THC or synthetic cannabinoids JWH-018 and JWH-073 induces tolerance to hypothermia but not locomotor suppression in mice, and reduces CB1 receptor expression and function in a brain region-specific manner. Pharmacol Res 102:22–32
Tseng AH, Craft RM (2001) Sex differences in antinociceptive and motoric effects of cannabinoids. Eur J Pharmacol 430(1):41–47
Varvel SA, Wiley JL, Yang R, Bridgen DT, Long K, Lichtman AH, Martin BR (2006) Interactions between THC and cannabidiol in mouse models of cannabinoid activity. Psychopharmacology 186(2):226–234
Wakley AA, Wiley JL, Craft RM (2014) Sex differences in antinociceptive tolerance to delta-9-tetrahydrocannabinol in the rat. Drug Alcohol Depend 143:22–28
Wall ME, Sadler BM, Brine D, Taylor H, Perez-Reyes M (1983) Metabolism, disposition, and kinetics of delta-9-tetrahydrocannabinol in men and women. Clin Pharmacol Ther 34(3):352–363
Wiley JL, Burston JJ (2014) Sex differences in Delta(9)-tetrahydrocannabinol metabolism and in vivo pharmacology following acute and repeated dosing in adolescent rats. Neurosci Lett 576:51–55
Wiley JL, Barrus DG, Farquhar CE, Lefever TW, Gamage TF (2021) Sex, species and age: effects of rodent demographics on the pharmacology of (9)-tetrahydrocanabinol. Prog Neuropsychopharmacol Biol Psychiatry 106:110064
Wiley JL, O'Connell MM, Tokarz ME, Wright MJ Jr (2007) Pharmacological effects of acute and repeated administration of Delta(9)-tetrahydrocannabinol in adolescent and adult rats. J Pharmacol Exp Ther 320(3), 1097-1105
Zgair A, Wong JC, Lee JB, Mistry J, Sivak O, Wasan KM, Hennig IM, Barrett DA, Constantinescu CS, Fischer PM, Gershkovich P (2016) Dietary fats and pharmaceutical lipid excipients increase systemic exposure to orally administered cannabis and cannabis-based medicines. Am J Transl Res 8(8):3448–3459
Funding
All experiments were supported by the National Institute on Drug Abuse of the National Institutes of Health grant numbers R21DA046154 (EW) and the Johns Hopkins University Dalio Fund in Decision Making and the Neuroscience of Motivated Behaviors (EW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to a Special Issue on Cannabis and Cannabinoids
Rights and permissions
About this article
Cite this article
Moore, C.F., Weerts, E.M. Cannabinoid tetrad effects of oral Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in male and female rats: sex, dose-effects and time course evaluations. Psychopharmacology 239, 1397–1408 (2022). https://doi.org/10.1007/s00213-021-05995-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-021-05995-5