Abstract
Rationale
Humans typically self-administer cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC) together repeatedly (as in cannabis, cannabis extract, or Sativex®) to relieve pain. It has been suggested that one benefit of the drug combination may be decreased tolerance development.
Objective
The present study compared the development of tolerance to the antinociceptive effects of THC given alone versus combined with CBD, in rats.
Methods
THC dose-effect curves on tail withdrawal and paw pressure tests were obtained before and after twice-daily treatment with vehicle or CBD (10 mg/kg), plus vehicle or THC (3.6 mg/kg females; 9.3 mg/kg males) for 4 days.
Results
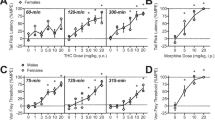
On the first day, THC was more potent in females than males on both nociceptive tests. From pre- to post-chronic (day 1 to day 6), THC potency on the tail withdrawal test decreased more in females than males, and rats that had been treated with CBD + THC repeatedly showed greater rightward/downward shifts of the THC dose-effect curve than rats that had been treated with THC alone. Analysis of blood samples taken after day 6 testing showed that serum THC levels were higher in CBD + THC-treated females than in vehicle + THC-treated females, and THC’s active metabolite 11-OH-THC and its inactive metabolite THC-COOH were lower in CBD + THC-treated rats than in vehicle + THC-treated rats of both sexes. CBD also increased serum levels of the active metabolite cannabinol in both sexes.
Conclusion
The decrease in THC’s antinociceptive effects after repeated CBD exposure may be due to CBD-induced inhibition of THC metabolism, and/or antagonism of THC effects that emerges with repeated CBD treatment.







Similar content being viewed by others
References
Amaya F, Shimosato G, Kawasaki Y, Hashimoto S, Tanaka Y, Ji RR, Tanaka M (2006) Induction of CB1 cannabinoid receptor by inflammation in primary afferent neurons facilitates antihyperalgesic effect of peripheral CB1 agonist. Pain 124:175–183
Booker L, Nauidu PS, Razadan RK, Mahadevan A, Lichtman AH (2009) Evaluation of prevalent phytocannabinoids in the acetic acid model of visceral nociception. Drug Alcohol Depend 105:42–47
Bornheim LM, Grillo MP (1998) Characterization of cytochrome P450 3A inactivation by cannabidiol: possible involvement of cannabidiol-hydroxyquinone as a P450 inactivator. Chem Res Toxicol 11:1209–1216
Bornheim LM, Kim KY, Li J, Perotti BY, Benet LZ (1995) Effect of cannabidiol pretreatment on the kinetics of tetrahydrocannabinol metabolites in mouse brain. Drug Metab Dispos 23:825–831
Brederson J-D, Kym PR, Szallasi A (2013) Targeting TRP channels for pain relief. Eur J Pharmacol 716:61–76
Britch SC, Wiley JL, Yu Z, Clowers BH, Craft RM (2017) Cannabidiol-delta-9-tetrahydrocannbinol interactions on acute pain and locomotor activity. Drug Alcohol Depend 175:187–197
Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selley LJ (2010) Regional enhancement of cannabinoid CB1 receptor desensitization in female adolescent rats following repeated Δ9-tetrahydrocannabinol exposure. Br J Pharmacol 161:103–112
Casey SL, Atwal N, Vaughn CW (2017) Cannabis constituent synergy in a mouse neuropathic pain model. Pain 158:2452–60
Cook CD, Barrett AC, Roach EL, Bowman JR, Picker MJ (2000) Sex-related differences in the antinociceptive effects of opioids: importance of rat genotype, nociceptive stimulus intensity, and efficacy at the mu opioid receptor. Psychopharmacology 150:430–442
Corchero J, Fuentes JA, Manzanares J (2002) Gender differences in proenkephalin gene expression response to Δ9-tetrahydrocannabinol in the hypothalamus of the rat. J Psychopharmacol 16:283–289
Costa B, Trovato AE, Comelli F, Giagnoni G, Colleoni M (2007) The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol 556:75–83
Craft RM, Wakley AA, Tsutsui KT, Laggart JD (2012) Sex differences in cannabinoid 1 vs. cannabinoid 2 receptor-selective antagonism of antinociception produced by delta9-tetrahydrocannabinol and CP55,940 in the rat. J Pharmacol Exp Ther 340:787–800
Cristino L, Palomba L, Di Marzo V (2014) New horizons on the role of cannabinoid CB1 receptors in palatable food intake, obesity and related dysmetabolism. Int J Obesity Suppl 4:S26–S30
Cuttler C, Mischley LK, Sexton M (2016) Sex differences in cannabis use and effects: a cross-sectional survey of cannabis users. Cannabis Cannabinoid Res 1:166–175
Dalton WS, Martz R, Lemberger L, Rodda BE, Forney RB (1976) Influence of cannabidiol on delta-9-tetrahydrocannabinol effects. Clin Pharmacol Ther 19:300–309
De Petrocellis L, Ligresti A, Moriello AS, Allara M, Bisogno T, Petrosino S, Stott CG, Di Marzo V (2011) Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol 163:1479–1494
Drewnowski A, Grinker JA (1978) Food and water intake, meal patterns and activity of obese and lean Zucker rats following chronic and acute treatment with delta9-tetrahydrocannabinol. Pharmacol Biochem Behav 9:619–630
Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W (2007) Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br J Pharmacol 152:795–804
Finn DP, Beckett SR, Roe CH, Madjd A, Fone KC, Kendall DA, Marsden CA, Chapman V (2004) Effects of coadministration of cannabinoids and morphine on nociceptive behaviour, brain monoamines and HPA axis activity in a rat model of persistent pain. Eur J Neurosci 19:678–686
Guide for the Care and Use of Laboratory Animals, 8th edition. Washington (DC): National Academies Press, 2011
Haney M, Malcolm RJ, Babalonis S, Nuzzo PA, Cooper ZD, Bedi G, Gray KM, McRae-Clark A, Lofwall MR, Sparenborg S, Walsh SL (2016) Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology 41:1974–1982
Harte-Hargrove LC, Dow-Edwards DL (2012) Withdrawal from THC during adolescence: sex differences in locomotor activity and anxiety. Behav Brain Res 231:48–59
Hiltunen AJ, Jarbe TUC, Wangdahl K (1988) Cannabinol and cannabidiol in combination: temperature, open-field activity, and vocalization. Pharmacol Biochem Behav 30:675–678
Hindocha C, Freeman TP, Schafer G, Gardener C, Das RK, Morgan CJ, Curran HV (2015) Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: a randomised, double-blind, placebo-controlled study in cannabis users. Eur Neuropsychopharmacol 25:325–334
Hložek T, Uttl L, Kadeřábek L, Balíková M, Lhotková E, Horsley RR, Nováková P, Šichová K, Štefková K, Tylš F, Kuchař M, Páleníček T (2017) Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur Neuropsychopharmacol 27:1223–1237
Holland ML, Lau DT, Allen JD, Arnold JC (2007) The multidrug transporter ABCG2 (BCRP) is inhibited by plant-derived cannabinoids. Br J Pharmacol 152:815–824
Ilan AB, Gevins A, Coleman M, ElSohly MA, de Wit H (2005) Neurophysiological and subjective profile of marijuana with varying concentrations of cannabinoids. Behav Pharmacol 16:487–496
Jacobs DS, Kohut SJ, Jiang S, Nikas SP, Makriyannis A, Bergman J (2016) Acute and chronic effects of cannabidiol and Δ9-tetrahydrocannabinol (Δ9-THC)-induced disruption in stop signal task performance. Exp Clin Psychopharmacol 24:320–330
Jaeger W, Benet LZ, Bornheim LM (1996) Inhibition of cyclosporine and tetrahydrocannabinol metabolism by cannabidiol in mouse and human microsomes. Xenobiotica 26:275–284
Jiang R, Yamaori S, Okamoto Y, Yamamoto I, Watanabe K (2013) Cannabidiol is a potent inhibitor of the catalytic activity of cytochrome P450 2C19. Drug Metab Pharmacokinet 28:332–338
Jones G, Pertwee RG (1972) A metabolic interaction in vivo between cannabidiol and 1-tetrahydrocannabinol. Br J Pharmacol 45:375–377
Karniol IG, Carlini EA (1973) Pharmacological interaction between cannabidiol and delta 9-tetrahydrocannabinol. Psychopharmacologia 33:53–70
Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA (1974) Cannabidiol interferes with the effects of Δ9-tetrahydrocannabinol in man. Eur J Pharmacol 28:172–177
Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA (2011) Plasma cannabinoid pharmacokinetics following controlled oral delta9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem 57:66–75
Kim H, Neubert JK, San Miguel A, Xu K, Krishnaraju RK, Iadarola MJ, Goldman D, Dionne RA (2004) Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain 109:488–496
King KM, Myers AM, Soroka-Monzo AJ, Tuma RF, Tallarida RJ, Walker EA, Ward SJ (2017) Single and combined effects of Δ9-tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy-induced neuropathic pain. Br J Pharmacol 174: 2832–41
Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, Gunasekaran N, Karl T, Long LE, Huang XF, Liu K, Arnold JC, McGregor IS (2011) Cannabidiol potentiates Δ9-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology 218:443–457
Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM (2015) Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol 172:4790–4805
Le Foll B, Trigo JM, Sharkey KA, Le Strat Y (2013) Cannabis and Δ9-tetrahydrocannabinol (THC) for weight loss? Med Hypotheses 80:564–567
Levendal RA, Schumann D, Donath M, Frost CL (2012) Cannabis exposure associated with weight reduction and β-cell protection in an obese rat model. Phytomedicine 19:575–582
Long LE, Chesworth R, Huang X, McGregor IS, Arnold JC, Karl T (2010) A behavioural comparison of acute and chronic Δ9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. Int J Neuropsychopharmacol 13:861–876
Marusich JA, Lefever TW, Antonazzo KR, Craft RM, Wiley JL (2014) Evaluation of sex differences in cannabinoid dependence. Drug Alcohol Depend 137:20–28
Nadulski T, Pragst F, Weinberg G, Roser P, Schnelle M, Fronk EM, Stadelmann AM (2005) Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of delta9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Ther Drug Monit 27:799–810
Narimatsu S, Watanabe K, Matsunaga T, Yamamoto I, Imaoka S, Funae Y, Yoshimura H (1990) Inhibition of hepatic microsomal cytochrome P450 by cannabidiol in adult male rats. Chem Pharm Bull 38:1365–1368
National Research Council (2011) Guide for the Care and Use of Laboratory Animals, 8th edition. National Academies Press, Washington (DC)
Riebe CJN, Hill MN, Lee TTY, Hillard CJ, Gorzalka BB (2010) Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology 35:1265–1269
Romero EM, Fernández B, Sagredo O, Gomez N, Urigüen L, Guaza C, De Miguel R, Ramos JA, Viveros MP (2002) Antinociceptive, behavioural and neuroendocrine effects of CP 55,940 in young rats. Brain Res Dev Brain Res 136:85–92
Russo E, Guy GW (2006) A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses 66:234–246
Sanders J, Jackson DM, Starmer GA (1979) Interactions among the cannabinoids in the antagonism of the abdominal constriction response in the mouse. Psychopharmacology 61:281–285
Sim-Selley LJ (2003) Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol 15:91–119
Sofia RD, Vassar HB, Knobloch LC (1975) Comparing analgesic activity of various naturally occurring cannabinoids in rats and mice. Psychopharmacologia 40:285–295
Tang SL, Tran V, Wagner EJ (2005) Sex differences in the cannabinoid modulation of an A-type K+ current in neurons of the mammalian hypothalamus. J Neurophysiol 94:2983–2986
Terner JM, Lomas LM, Smith ES, Barrett AC, Picker MJ (2003) Pharmacogenetic analysis of sex differences in opioid antinociception in rats. Pain 106:381–391
Todd SM, Zhou C, Clarke DJ, Chohan TW, Bahceci D, Arnold JC (2017) Interactions between cannabidiol and Δ9-THC following acute and repeated dosing: rebound hyperactivity, sensorimotor gating and epigenetic and neuroadaptive changes in the mesolimbic pathway. Eur Neuropsychopharmacol 27:132–145
Tseng AH, Craft RM (2001) Sex differences in antinociceptive and motoric effects of cannabinoids. Eur J Pharmacol 430:41–47
Tseng AH, Harding JW, Craft RM (2004) Pharmacokinetic factors in sex differences in delta 9-tetrahydrocannabinol-induced behavioral effects in rats. Behav Brain Res 154:77–83
Varvel SA, Wiley JL, Yang R, Bridgen DT, Long K, Lichtman AH, Martin BR (2006) Interactions between THC and cannabidiol in mouse models of cannabinoid activity. Psychopharmacology 186:226–234
Villares J (2007) Chronic use of marijuana decreases cannabinoid receptor binding and mRNA expression in the human brain. Neuroscience 145:323–334
Wakley AA, Wiley JL, Craft RM (2014) Sex differences in antinociceptive tolerance to delta-9-tetrahydrocannabinol in the rat. Drug Alcohol Depend 143:22–28
Wakley AA, Wiley JL, Craft RM (2015) Gonadal hormones do not alter the development of antinociceptive tolerance to delta-9-tetrahydrocannabinol in adult rats. Pharmacol Biochem Behav 133:111–121
Watanabe K, Yamaori S, Funahashi T, Kimura T, Yamamoto I (2007) Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life Sci 80:1415–1419
Wiley JL, Burston JJ (2014) Sex differences in Δ(9)-tetrahydrocannabinol metabolism and in vivo pharmacology following acute and repeated dosing in adolescent rats. Neurosci Lett 576:51–55
Wiley JL, O'Connell MM, Tokarz ME, Wright MJ (2007) Pharmacological effects of acute and repeated administration of delta(9)-tetrahydrocannabinol in adolescent and adult rats. J Pharmacol Exp Ther 320:1097–1105
Yamaori S, Kushihara M, Yamamoto I, Watanabe K (2010) Characterization of major phytocannabinoids, cannabidiol and cannabinol, as isoform-selective and potent inhibitors of human CYP1 enzymes. Biochem Pharmacol 79:1691–1698
Yamaori S, Okamoto Y, Yamamoto I, Watanabe K (2011) Cannabidiol, a major phytocannabinoid, as a potent atypical inhibitor for CYP2D6. Drug Metab Dispos 39:2049–2056
Zhu HJ, Wang JS, Markowitz JS, Donovan JL, Gibson BB, Gefroh HA, Devane CL (2006) Characterization of P-glycoprotein inhibition by major cannabinoids from marijuana. J Pharmacol Exp Ther 317:850–857
Zuardi AW, Hallak JE, Crippa JA (2012) Interaction between cannabidiol (CBD) and ∆(9)-tetrahydrocannabinol (THC): influence of administration interval and dose ratio between the cannabinoids. Psychopharmacology 219:247–249
Acknowledgements
The authors thank Kelly Hewitt and Abby Pondelick for excellent technical assistance.
Funding
This research was funded by NIDA DA016644 (J. Wiley, PI), by a Diversity Supplement to DA016644 (to support N. Greene), and by funds dedicated for marijuana research by the State of Washington Initiative Measure 502.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Greene, N.Z., Wiley, J.L., Yu, Z. et al. Cannabidiol modulation of antinociceptive tolerance to Δ9-tetrahydrocannabinol. Psychopharmacology 235, 3289–3302 (2018). https://doi.org/10.1007/s00213-018-5036-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-5036-z