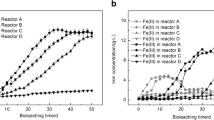
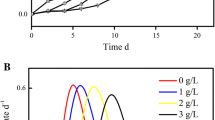
Abstract
In Acidithiobacillus ferrooxidans, one of the most important bioleaching bacterial species, the proteins encoded by the rus operon are involved in the electron transfer from Fe2+ to O2. To obtain further knowledge about the mechanism(s) involved in the adaptive responses of the bacteria to growth on the different uranium ore pulp densities, we analyzed the expression of the four genes from the rus operon by real-time PCR, when Acidithiobacillus sp. FJ2 was grown in the presence of different uranium concentrations. The uranium bioleaching results showed the inhibitory effects of the metal pulp densities on the oxidation activity of the bacteria which can affect Eh, pH, Fe oxidation and uranium extractions. Gene expression analysis indicated that Acidithiobacillus sp. FJ2 tries to survive in the stress with increasing in the expression levels of cyc2, cyc1, rus and coxB, but the metal toxicity has a negative effect on the gene expression in different pulp densities. These results indicated that Acidithiobacillus sp. FJ2 could leach the uranium even in high pulp density (50%) by modulation in rus operon gene responses.



Similar content being viewed by others
References
Abhilash S, Pandey BD (2011) Role of ferric ions in bioleaching of uranium from low tenor Indian ore. Can Metall Q 50(2):102–112. doi:10.1179/000844311X12949291728050
Abhilash S, Mehta KD, Kumar V, Pandey DB, Tamrakar KP (2011) Bioleaching—an alternate uranium ore processing technology for India. Energy Proced 7:158–162. doi:10.1016/j.egypro.2011.06.021
Almárcegui RJ, Navarro CA, Paradela A, Albar JP, Bernath DV, Jerez CA (2014) Response to copper of Acidithiobacillus ferrooxidans ATCC 23270 grown in elemental sulfur. Res Microbiol 165(9):761–772. doi:10.1016/j.resmic.2014.07.005
Appia-Ayme C, Guiliani N, Ratouchniak J, Bonnefoy V (1999) Characterization of an operon encoding two c-type cytochromes, an aa3-type cytochrome oxidase, and rusticyanin in Thiobacillus ferrooxidans ATCC 33020. Appl Environ Microbiol 65:4781–4787
Baker BJ, Banfield JF (2003) Microbial communities in acid mine drainage. FEMS Microbiol Ecol 44:139–152. doi:10.1016/S0168-6496(03)00028-X
Bengrine A, Guiliani N, Appia-Ayme C, Jedlicki E, Holmes DS, Chippaux M, Bonnefoy V (1998) Sequence and expression of the rusticyanin structural gene from Thiobacillus ferrooxidans ATCC 33020 strain. Biochim Biophys Acta 1443:99–112. doi:10.1016/S0167-4781(98)00199-7
Bruscella P, Appia-Ayme C, Levica´n G, Ratouchniak J, Jedlicki E, Holmes DS, Bonnefoy V (2007) Differential expression of two bc1 complexes in the strict acidophilic chemolithoautotrophic bacterium Acidithiobacillus ferrooxidans suggests a model for their respective roles in iron or sulfur oxidation. Microbiology 153:102–110. doi:10.1099/mic.0.2006/000067-0
Carlos C, Reis FC, Vicentini R, Madureira DJ, Ottoboni LMM (2008) The rus operon genes are differentially regulated when Acidithiobacillus ferrooxidans LR is kept in contact with metal sulfides. Curr Microbiol 57:375–380. doi:10.1007/s00284-008-9208-7
Debabrata P, Dong J, Kim J, Seoung W (2010) Microbial leaching process to recover valuable metals from spent petroleum catalyst using iron oxidizing bacteria. World Acad Sci Eng Technol 4:02–22
Dekker L, Arsene-Ploetze F, Santini JM (2016) Comparative proteomics of Acidithiobacillus ferrooxidans grown in the presence and absence of uranium. Res Microbiol 167:234–239. doi:10.1016/j.resmic.2016.01.007
Dong Y, Lin H, Wang H, Mo X (2011) Effects of ultraviolet irradiation on bacteria mutation and bioleaching of low-grade copper tailings. Crit Rev Microbiol Miner Eng 24:870–875. doi:10.1016/j.mineng.2011.03.020
Fatemi F, Rashidi A, Jahani S (2015) Isolation and Identification of native sulfur-oxidizing bacterium capable of uranium extraction. J Sci Univ Tehran Prog Biol Sci 5:207–221
Fatemi F, Arabieh M, Jahani S (2016) Application of response surface methodology to optimize uranium biological leaching at high pulp density. Radiochim Acta 104(4):239–246. doi:10.1515/ract-2015-2495
Fatemi F, Jahani S, Miri S (2017) The effect of peptone and tryptic soy broth (TSB) on uranium bioleaching efficiency. J Nucl Sci Technol (in press)
Giudici-Orticoni MT, Guerlesquin F, Bruschi M, Nitschke W (1999) Interaction-induced redox switch in the electron transfer complex rusticyanin-cytochrome c 4. J Biol Chem 274:30365–30369. doi:10.1074/jbc.274.43.30365
Golmohammadi H, Rashidi A, Safdari SJ (2012) Simple and rapid spectrophotometric method for determination of uranium(VI) in low grade uranium ores using arsenazo(III). Chem Chem Technol 6:245–249
Gomez E, Ballester A, Gonzalez F, Blazquez ML (1999) Leaching capacity of a new extremely thermophilic microorganism, Sulfolobus rivotincti. Hydrometallurgy 52:349–366. doi:10.1016/S0304-386X(99)00027-4
Ingledew WJ (1982) Thiobacillus ferrooxidans the bioenergetics of an acidophilic chemolithotroph. Biochim Biophys Acta 683:89–117
Jahani S, Fatemi F, Firoz-e-zare MA, Zolfaghari MR (2015) Isolation and characterization of Acidithiobacillus ferrooxidans strain FJS from Ramsar, Iran. Electron J Biol 11(4):138–146
Johnson DB, Hallberg KB (2003) The microbiology of acidic mine waters. Res Microbiol 154:466–473. doi:10.1016/S0923-2508(03)00114-1
Karamanev DG, Nikolov LN, Mamatarkova V (2002) Rapid simultaneous quantitative determination of ferric and ferrous ions in drainage waters and similar solutions. Miner Eng 15(5):341–346. doi:10.1016/S0892-6875(02)00026-2
Kucera J, Bouchal P, Lochman J, Potesil D, Janiczek O, Zdrahal Z, Mandl M (2013) Ferrous iron oxidation by sulfur-oxidizing Acidithiobacillus ferrooxidans and analysis of the process at the levels of transcription and protein synthesis. Antonie Van Leeuwenhoek 103:905–919. doi:10.1007/s10482-012-9872-2
Levica´n G, Bruscella P, Guacucano M, Inostroza C, Bonnefoy V, Holmes DS, Jedlicki E (2002) Characterization of the petI and res operons of Acidithiobacillus ferrooxidans. J Bacteriol 184:1498–1501. doi:10.1128/JB.184.5.1498-1501
Liang G, Tang J, Liu W, Zhou Q (2013) Optimizing mixed culture of two acidophiles to improve copper recovery from printed circuit boards (PCBs). J Hazard Mater 15:250–251. doi:10.1016/j.jhazmat.2013.01.077
Lottering JM, Lorenzen L, Phala SN, Smit TJ, Schakwyk CAG (2008) Mineralogy and uranium leaching response of low grade South African ores. Miner Eng 21:16–22. doi:10.1016/j.mineng.2007.06.006
Matlakowska R, Sklodowska A (2006) Adaptive responses of chemolithoautotrophic acidophilic Acidithiobacillus ferrooxidans to sewage sludge. J Appl Microbiol 12:1485–1498. doi:10.1111/j.1365-2672.2006.03208.x
Mei Li H, Jun Ke J (2001) Technical note in influence of Cu2+ and Mg2+ on the growth and activity of Ni2+ adapted Thiobacillus ferrooxidans. Miner Eng 14:113–116. doi:10.1016/S0892-6875(00)00165-5
Munoz JA, Gonzales F, Blazquez ML, Ballester A (1995a) A study of the bioleaching of a Spanish uranium ore, part I: a review of the bacterial leaching in the treatment of uranium ores. Hydrometallurgy 38:39–57
Munoz JA, Ballester A, Gonzalez F, Blazquez ML (1995b) A study of the bioleaching of spanish uranium ore part II. Orbital shaker experiments. Hydrometallurgy 38:59–78
Mykytczuk NCS, Trevors JT, Ferroni GD, Leduc LG, Foote SJ, Twine SM (2011) Proteomic insights into cold adaptation of psychrotrophic and mesophilic Acidithiobacillus ferrooxidans strains. Antonie Van Leeuwenhoek 100:259–277. doi:10.1007/s10482-011-9584-z
Patel M, Tipre D, Dave S (2011) Isolation, identification, characterization and polymetallic concentrate leaching studies of tryptic soy- and peptone-resistant thermotolerant Acidithiobacillus ferrooxidans SRDSM2. Bioresour Technol 102:1602–1607. doi:10.1016/j.biortech.2010.08.115
Ramírez P, Guiliani N, Valenzuela L, Beard S, Jerez CA (2004) Differential protein expression during growth of Acidithiobacillus ferrooxidans on ferrous iron, sulfur compounds, or metal sulfides. Appl Environ Microbiol 70(8):4491–4498. doi:10.1128/AEM.70.8.4491-4498.2004
Rawlings DE (2005) Characteristics and adaptability of iron- and sulfuroxidizing microorganisms used for the recovery of metals from minerals and their concentrates. Microb Cell Fact 4:13. doi:10.1186/1475-2859-4-13
Schippers A, Sand W (1999) Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur. Appl Environ Microbiol 65:319–321
Silver M, Margalith P, Lundgren DG (1967) Effect of glucose on carbon dioxide assimilation and substrate oxidation by Ferrobacillus ferrooxidans. J Bacteriol 77:1765–1769
Tuovinen OH, Bhatti TM, Bigham JM, Hallberg KB, Garcia O, Lindstrom EB (1994) Oxidative dissolution of arsenopyrite by mesophilic and moderately thermophilic acidophiles. Appl Environ Microbiol 60:32–68
Tuttle JH, Dugan PR (1976) Inhibition of growth, iron and sulphur oxidation in Thiobacillus ferrooxidans by simple organic compounds. Can J Microbiol 22:719–730
Xue-ling W, Guan-zhou Q, Jian G, Jian-nan D, Jian K, Xin-xing L (2007) Mutagenic breeding of silver-resistant Acidithiobacillus ferrooxidans and exploration of resistant mechanism. Trans Nonferrous Met Soc China 17:412–417. doi:10.1016/S1003-6326(07)60107-1
Yarzábal A, Brasseur G, Ratouchniak J, Lund K, Lemesle-Meunier D, DeMoss JA, Bonnefoy V (2002a) The high-molecular-weight cytochrome c cyc2 of Acidithiobacillus ferrooxidans is an outer membrane protein. J Bacteriol 184:313–317. doi:10.1128/JB.184.1.313-317.2002
Yarzábal A, Brasseur G, Bonnefoy V (2002b) Cytochromes c of Acidithiobacillus ferrooxidans. FEMS Microbiol Lett 209:189–195. doi:10.1111/j.1574-6968.2002.tb11130.x
Yarzábal A, Duquesne K, Bonnefoy V (2003) Rusticyanin gene expression of Acidithiobacillus ferrooxidans ATCC 33020 in sulfur- and in ferrous iron media. Hydrometallurgy 71:107–114
Yarzábal A, Appia-Ayme C, Ratouchniak J, Bonnefoy V (2004) Regulation of the expression of the Acidithiobacillus ferrooxidans rus operon encoding two cytochromes c, a cytochrome oxidase and rusticyanin. Microbiology 150:2113–2123. doi:10.1099/mic.0.26966-0
Yu R, Tan J, Gu G, Hu Y, Qiu G (2010) Mechanism of bioleaching chalcopyrite by Acidithiobacillus ferrooxidansin agar-simulated extracellular polymeric substances media. J Cent South Univ Technol 17:56–61. doi:10.1007/s11771-010-0011-9
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Harald Huber.
Rights and permissions
About this article
Cite this article
Fatemi, F., Miri, S. & Jahani, S. Effect of metal sulfide pulp density on gene expression of electron transporters in Acidithiobacillus sp. FJ2. Arch Microbiol 199, 521–530 (2017). https://doi.org/10.1007/s00203-016-1318-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-016-1318-1