Abstract
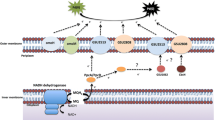
Acidithiobacillus ferrooxidans is a Gram-negative bacterium that obtains energy from the oxidation of ferrous iron or reduced sulfur compounds. In this bacterium, the proteins encoded by the rus operon are involved in electron transfer from Fe(II) to O2, and the first two proteins in this pathway also participate in the electron transfer pathway from Fe(II) to NAD(P). In this work we analyzed the expression, by real-time PCR, of the eight genes from the rus operon when A. ferrooxidans LR was grown in the presence of iron (control) and then kept in contact with chalcopyrite (CuFeS2) and covellite (CuS). A small decrease in rus operon gene expression was observed in the presence of chalcopyrite, while in the presence of covellite the expression of these genes showed a remarkable decrease. These results can be explained by the absence of ferrous iron in covellite. To explain the expression difference observed between the gene cyc1 and the gene rus, we investigated the information content presented at the Translation Initiation Site (TIS) of both genes. cyc1 showed a highly information content (8.4 bits) that can maximize translation, and rus showed a less favorable context (5.5 bits). Our hypothesis is that the energetic metabolism in A. ferrooxidans may be controlled at the transcriptional and posttranscriptional level by different mechanisms.

Similar content being viewed by others
References
Appia-Ayme C, Guiliani N, Ratouchniak J, Bonnefoy V (1999) Characterization of an operon encoding two c-type cytochromes, an aa 3-type cytochrome oxidase and rusticyanin in Thiobacillus ferrooxidans ATCC 33020. Appl Environ Microbiol 65:4781–4787
Bengrine A, Guiliani N, Appia-Ayme C, Jedlicki E, Holmes DS, Chippaux M, Bonnefoy V (1998) Sequence and expression of the rusticyanin structural gene from Thiobacillus ferrooxidans ATCC 33020 strain. Biochim Biophys Acta 1443:99–112
Brasseur G, Levican G, Bonnefoy V, Holmes D, Jedlicki E, Lemesle-Meunier D (2004) Apparent redundancy of electron transfer pathways via bc(1) complexes and terminal oxidases in the extremophilic chemolithoautotrophic Acidithiobacillus ferrooxidans. Biochim Biophys Acta 1656:114–126
Bruscella P, Appia-Ayme C, Levicán G, Ratouchniak J, Jedlicki E, Holmes DS, Bonnefoy V (2007) Differential expression of two bc 1 complexes in the strict acidophilic chemolithoautotrophic bacterium Acidithiobacillus ferrooxidans suggests a model for their respective roles in iron or sulfur oxidation. Microbiology 153:102–110
Elbehti A, Brasseur G, Lemesle-Meunier D (2000) First evidence for existence of an uphill electron transfer through the bc 1 and NADH-Q oxidoreductase complexes of the acidophilic obligate chemolithotrophic ferrous iron-oxidizing bacterium Thiobacillus ferrooxidans. J Bacteriol 182:3602–3606
Garcia O Jr (1991) Isolation and purification of Thiobacillus ferrooxidans and Thiobacillus thiooxidans from some coal and uranium mines of Brazil. Revista Microbiol 22:1–6
Hauryliuk V, Ehrenberg M (2006) Two-step selection of mRNAs in initiation of protein synthesis. Mol Cell 22:155–156
Holmes DS, Bonnefoy V (2007) Genetic and bioinformatics insights into iron and sulfur oxidation mechanisms of bioleaching organisms. In: Rawlings DE, Johnson BD (eds) Biomining. Springer-Verlag, New York, pp 281–307
Ingledew WJ (1982) T. ferrooxidans, the bioenergetics of an acidophilic chemolithotrophic bacteria. Biochim Biophys Acta 683:89–117
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔC(T) method. Methods 25:402–408
Quatrini R, Appia-Ayme C, Denis Y et al (2006) Insights into the iron and sulfur energetic metabolism of Acidithiobacillus ferrooxidans by microarray transcriptome profiling. Hydrometallurgy 83:263–272
Ramirez P, Guiliani N, Valenzuela L, Beard S, Jerez CA (2004) Differential protein expression during growth of Acidithiobacillus ferrooxidans on ferrous iron, sulfur compounds, or metal sulfides. Appl Environ Microbiol 70:4491–4498
Rawlings DE (2002) Heavy metal mining using microbes. Annu Rev Microbiol 56:65–91
Steitz JA, Jakes K (1975) How ribosomes select initiator regions in mRNA: base pair formation between the 3’ terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci USA 72:4734–4738
Tuovinen OH, Kelly DP (1972) Biology of Thiobacillus ferrooxidans in relation to the microbiological leaching of sulphide ore. Z Allg Mikrobiol 12:311–346
Vicentini R, Menossi M (2007) TISs-ST: a web server to evaluate polymorphic translation initiation sites and their reflections on the secretory targets. BMC Bioinform 8:160
Winderickx J, Castro JM (1994) Pratical course in molecular biology of microorganisms. Universidade Federal de Ouro Preto-MG, January 23–February 11, p 59
Yarzábal A, Brasseur G, Ratouchniak J, Lund K, Lemesle-Meunier D, DeMoss JA, Bonnefoy V (2002) The high molecular weight cytochrome c Cyc2 of Acidithiobacillus ferrooxidans is an outer membrane protein. J Bacteriol 184:313–317
Yarzábal A, Appia-Ayme C, Ratouchniak J, Bonnefoy V (2004) Regulation of the expression of the Acidithiobacillus ferrooxidans rus operon encoding two cytochromes c, a cytochrome oxidase and rusticyanin. Microbiology 150:2113–2123
Acknowledgments
This work was supported by a grant from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2005/51332-7). L.M.M.O. received a research fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). C.C. (06/54433-1) and F.C.R. (05/00139-2) received fellowships from FAPESP. D.J.M. (134062/2006-0) received a fellowship from CNPq. The authors thank Dr. O. Garcia Jr., for providing A. ferrooxidans strain LR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carlos, C., Reis, F.C., Vicentini, R. et al. The rus Operon Genes Are Differentially Regulated When Acidithiobacillus ferrooxidans LR is Kept in Contact with Metal Sulfides. Curr Microbiol 57, 375–380 (2008). https://doi.org/10.1007/s00284-008-9208-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-008-9208-7