Abstract
Summary
This study evaluated the cost-effectiveness of sequential treatment with romosozumab-to-alendronate compared to alendronate monotherapy and teriparatide-to-alendronate, in postmenopausal osteoporotic women from a Belgian healthcare perspective. Romosozumab-to-alendronate was found to be cost-effective compared to alendronate monotherapy and dominant compared to teriparatide-to-alendronate for osteoporotic women at high risk of fracture in Belgium.
Purpose
This study aimed to evaluate the cost-effectiveness of sequential treatment with romosozumab followed by alendronate compared to alendronate monotherapy and teriparatide followed by alendronate, in postmenopausal osteoporotic women at high risk of fracture, from a Belgian healthcare perspective. Romosozumab is reimbursed in Belgium since December 2021.
Methods
A Markov microsimulation model was used to evaluate the cost-effectiveness of romosozumab-to-alendronate compared to alendronate monotherapy and to teriparatide-to-alendronate over a lifetime horizon. Patients transition between five different health states every 6 months based on fracture risks or death. The model was populated with Belgium-specific epidemiological and cost data, where available. The fracture risk reduction of romosozumab treatment was collated from the ARCH study, and from a published network meta-analysis. Costs were included from a healthcare perspective (NIHDI). Cost-effectiveness was reported in terms of costs per quality-adjusted life year (QALY), reported in Euro (€) 2022. Deterministic (DSA) and probabilistic sensitivity analyses (PSA) were performed.
Results
Romosozumab-to-alendronate was associated with 0.12 additional QALYs at an additional cost of €2314 compared to alendronate monotherapy, resulting in an ICER of €19,978. Compared to teriparatide-to-alendronate, romosozumab-to-alendronate was found to be dominant, with higher QALYs and lower costs. The base-case results were robust to uncertainty in the input parameters when conducting the sensitivity analysis.
Conclusion
Sequential treatment with romosozumab followed by alendronate was found to be cost-effective compared to alendronate monotherapy and dominant compared to teriparatide followed by alendronate for postmenopausal women with osteoporosis at high risk of fracture in Belgium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The burden of osteoporosis is significant, both in economic and human terms. Globally, osteoporosis causes around 9 million new fractures each year, and this number is steadily increasing. It is projected to affect 200 million women worldwide, with one in ten women in their 60s, two in ten in their 70s, four in ten in their 80s, and seven in ten in their 90s being affected by osteoporosis [1]. In the total Belgian population, the prevalence of osteoporosis was found to be 5.6% in 2019, which is on par with the estimated average (5.6%) for the 27 countries of the European Union plus the UK and Switzerland (EU27 + 2) [2]. According to data from 2019, Belgium had the ninth highest per-capita cost of osteoporotic fractures among the EU27 + 2 countries [2, 3].
It is well established that a fragility fracture increases the risk of a subsequent fracture over a patient’s lifetime. In the first 2 years after an initial fragility fracture, the risk of subsequent fractures is highest, and within those 2 years, about 50% of all subsequent fractures occur [4]. This increased risk in the first 2 years following a fracture has been termed “imminent risk,” and these patients can be characterized as being at very high fracture risk [5]. High costs associated with inpatient and outpatient care, decreased quality of life, and a rise in mortality rate are major factors impacting the burden of recurrent fractures on both healthcare systems and patients. Regardless of this burden, individuals suffering from fragility fractures remain largely untreated [2].
Romosozumab, a bone-forming agent that exerts a dual effect on bone, increasing bone formation while decreasing bone resorption, was approved in Europe in 2019 for the treatment of severe osteoporosis in postmenopausal women at high risk of fracture [6]. It is recommended to use romosozumab in a treatment sequence to extend the benefit achieved with romosozumab beyond 12 months [6]. The treatment sequence includes administering 12 doses of 210 mg of romosozumab on a monthly basis, followed by an antiresorptive treatment [6]. A significant reduction in the risk of vertebral and non-vertebral fractures, including hip fractures, was reported in a phase III clinical trial, which compared monthly romosozumab vs. weekly alendronate in a blinded fashion for 12 months, followed by open-label alendronate in both groups up to 36 months in postmenopausal women with osteoporosis [7].
Compared to other treatment alternatives, as shown in a recently published network meta-analysis (NMA), romosozumab’s bone-forming ability offers potential to improve reduction in the risk of fracture among patients with recent fracture [8]. As such, sequential treatment could prevent new fractures more effectively when compared to conventional treatment approaches involving either antiresorptive treatment or bone-building treatment alone [5].
The Belgian Bone Club (BBC) treatment guideline recommends antiresorptive treatment in patients at high fracture risk, while bone-building therapy followed by antiresorptive therapy may be considered in patients at very high risk of fracture [5]. According to this guideline, patients with a recent major osteoporotic fracture (MOF), which is defined as a fragility fracture within the past 2 years of the spine, pelvis, hip, femur, humerus, and (in persons aged ≥ 75 years) forearm, could be considered at very high risk of fracture. Patients without a recent MOF are categorized in low or high fracture risk depending on their bone mineral density (BMD) and fracture risk based on clinical risk factors (e.g., calculated with the FRAX® fracture risk assessment tool).
Purpose
The objective of this study was to assess the cost-effectiveness of sequential romosozumab followed by alendronate compared to alendronate monotherapy and to teriparatide followed by alendronate in osteoporotic women with a recent fracture and thus at high risk of fracture from a Belgian healthcare payer perspective.
Methods
Model structure
A Markov microsimulation model was employed to estimate the cost-effectiveness of romosozumab-to-alendronate in comparison to alendronate monotherapy and teriparatide-to-alendronate. In a microsimulation model, each patient was evaluated individually, and a record of their previous health states was created within the model. The patients were tracked through health states, for which costs and benefits accumulated over time. These models are appropriate when many health states are relevant, and patients are assumed to be at a changing risk of incurring multiple events with long-term consequences, such as osteoporosis. The model has been previously described in detail by Söreskog et al. [9].
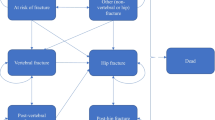
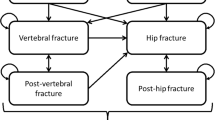
The model comprised five health states: “At risk of fracture,” “Hip fracture,” “Vertebral fracture,” “Non-hip, non-vertebral fracture (NHNV),” and “Death.” The model cycle length was 6 months, which is commonly used in cost-effectiveness models for osteoporosis treatments [10]. In the model, all patients began in the “At risk of fracture” health state, and at the end of each 6-month cycle, they had a probability of incurring new hip, vertebral, or NHNV fractures, remaining in a health state without a new fracture, or dying. In the case of death, the patient transitioned to the “Death” state and remained there for the remainder of the simulated time horizon, incurring no further events or costs (absorbing health state). Figure 4 in supplementary materials summarizes the health states and possible transitions.
Due to the chronic nature of osteoporosis, a lifetime horizon was chosen in the base-case analysis. All patients were followed on an individual basis from their age at start of treatment until the age of 100 years or death, whichever came first.
Target patient population
The target population for romosozumab was postmenopausal women with osteoporosis, who were at high risk of fracture. The inclusion criteria for this population were women aged 50 years and above, with a T-score of ≤ − 2.5 and a recent MOF, within the last 24 months. These population criteria are broadly aligned with the reimbursement criteria for romosozumab in Belgium which allow specialists in rheumatology, physiotherapy, and internal medicine including geriatricians to request reimbursement for romosozumab [11]. Age at treatment start for the base case was set at 74 years which was the mean age in the phase III clinical trial ARCH [7].
Treatment comparators
The model simultaneously compared sequential treatment of romosozumab (i.e., romosozumab treatment followed by antiresorptive treatment) with another active comparator arm. Romosozumab-to-alendronate was compared with alendronate monotherapy and with teriparatide followed by alendronate.
The antiresorptive treatment chosen to follow romosozumab is alendronate, as clinical evidence of alendronate following romosozumab is available from the ARCH trial [7]. Alendronate is also one of the cheapest osteoporosis treatments in Belgium without restrictive reimbursement criteria [11]. Alendronate was also the active comparator arm in the pivotal phase III study ARCH [7] and is recommended as treatment by the BBC treatment guideline for patients at high fracture risk [5]. Alendronate was therefore considered the most appropriate comparator.
Teriparatide was the first bone-building therapy approved for the treatment of severe osteoporosis and is recommended as second-line treatment for patients at very high fracture risk in Belgium [5]. The reimbursement criteria for teriparatide in Belgium include a maximum treatment length of 18 months [11].
Treatment duration and persistence
In the economic analyses, it was assumed that patients receiving romosozumab-to-alendronate treatment would be treated with romosozumab for a maximum of 1 year followed by 4 years of alendronate, whereas patients receiving alendronate monotherapy would remain on alendronate for a maximum of 5 years. Patients receiving teriparatide-to-alendronate treatment would be treated with teriparatide for a maximum of 1.5 years (in line with Belgian reimbursement criteria for teriparatide) followed by 3.5 years of alendronate [11]. Only patients who persisted with romosozumab for the initial year or with teriparatide for the initial 1.5 years were switched to alendronate.
The persistence to romosozumab in clinical practice is unknown. Treatment completion rates for the phase III clinical trial ARCH were approximately 90% at 12 months with romosozumab and approximately 77% at primary analysis with sequential alendronate [7]. Persistence for teriparatide was derived from a Swedish study with a 6-month and 12-month persistence of approximately 74% and 61%, respectively [12]. Since romosozumab is administered less frequently than teriparatide (monthly vs. daily), it was assumed that patients treated with romosozumab would have better persistence compared to those treated with teriparatide. The magnitude of the difference, however, is unknown. It was assumed that 90% of patients would persist throughout the 12-month treatment length. A scenario analysis was performed with persistence at 80%.
Since there is a lack of data on the persistence with alendronate as a follow-on therapy after bone-building agents, a DELPHI survey was conducted. Sixteen Belgian physicians with experience in treating osteoporosis participated in the survey [13]. According to the survey results, alendronate as a sequential therapy would have a persistence rate of 65% at year 1 and 30% at year 4. These rates were used for alendronate as a follow-on therapy after both romosozumab and teriparatide in the analysis. Sensitivity analyses using persistence rates sourced from Morley et al. [14] were also performed.
The persistence with alendronate as monotherapy was sourced from Li et al. [15]. Persistence for each time point and treatment is shown in Supplementary Table 5.
Fracture risk
The fracture risk in the model was based on a composite of three elements: the general population fracture incidence, the increased risk of fracture associated with severe osteoporosis (the relative risk) compared to the general population, and the effect of treatment on fracture risk.
Fracture incidence in the population
The incidences of hip fractures were obtained from a national hospital database that covers all annual hospital stays in Belgium [16]. Comprehensive data on the risk of clinical vertebral and NHNV fractures in Belgium are scarce. However, the proportionality between fracture types is believed to be similar across the Western world [17]. Therefore, the incidence of fractures in Belgium was estimated by assuming the same ratio of clinical vertebral and NHNV fractures to hip fractures as observed in several studies [17,18,19]. The age-adjusted incidence of fractures per 100,000 person-years in Belgium is presented in Supplementary Table 1.
Fracture risk estimation for target patient populations
The model was designed to accommodate both traditional and FRAX®-based risk assessment methods for calculating the relative risk of fractures for target patient populations compared to the general population risk, as described in Ström et al. [20]. The traditional method calculates relative risks using three clinical risk factors, namely age, BMD, and the prevalence of vertebral fractures [21], while the FRAX® tool takes into account ten clinical risk factors (CRFs) [22]. In estimating the relative risk using FRAX®, all CRFs need to be specified, whereas in the traditional method, all risk factors except age, BMD, and prevalent vertebral fracture are assumed to be prevalent at the same level as in the general population. Additionally, the traditional method can estimate relative risks below a certain T-score, whereas FRAX® estimates relative risk at a certain T-score. Since the two approaches reflect different types of patient populations, it is not always appropriate to compare their estimated relative risks.
In the base-case simulations, the traditional approach was used as it was submitted and accepted by the Belgian health technology assessment (HTA) authority for the reimbursement approval of romosozumab. However, a sensitivity analysis explored the FRAX®-based approach.
The model also captured the time-dependent increase in fracture risk after a fracture occurrence, which is not considered in either the traditional method or FRAX®. To estimate the time-dependent relative fracture risk in patients after a fracture, the model used algorithms based on Swedish retrospective data [23]. Figure 1 provides an example of how the fracture risk trajectory was estimated in the model using the traditional approach at different time points in a patient without a fracture at baseline.
Treatment efficacy
Efficacy of romosozumab-to-alendronate vs. alendronate monotherapy on the risk of hip, vertebral, and NHNV fractures was sourced from the phase III trial ARCH that studied the efficacy of romosozumab followed by alendronate compared with alendronate monotherapy [7]. Hazard ratios for romosozumab-to-alendronate vs. placebo were needed for the model because it was based on fracture risk for an untreated population. These were calculated by multiplying the hazard ratios of romosozumab-to-alendronate vs. alendronate monotherapy with hazard ratios of alendronate vs. placebo based on a published NMA [8]. The efficacy of teriparatide-to-alendronate has not been studied in a RCT as it has been for sequential romosozumab. Therefore, the efficacy of teriparatide to alendronate was derived from a NMA [8]. The observed cumulative effect in the NMA of teriparatide compared to placebo over 0–12 and 0–24 months and the cumulative effect of alendronate compared to placebo over 0–36 months was used for the teriparatide to alendronate treatment sequence. For alendronate as monotherapy, the cumulative effect over 0–12, 0–24, and 0–36 months compared to placebo was used. As data for 0–18 for teriparatide vs. placebo was not included in the NMA, 0–24 was conservatively used.
The treatment effects on fracture risk used in the model are described in Table 1. Treatment effect on fracture risk persists for a time (offset time) following treatment discontinuation, but few studies have directly evaluated the duration of offset after stopping treatment [24, 25]. The results of those studies vary but indicate that the residual efficacy may persist for at least as long as the time on treatment, except for denosumab. In line with previous research, following treatment discontinuation, treatment efficacy was assumed to linearly decline to zero over a period corresponding to the time a patient remained on treatment.
Mortality
The age and gender specific mortality rates for the general population in Belgium were based on the mortality rate in 2021 sourced from Statistics Belgium [26].
Hip and vertebral fractures are associated with an increase in mortality [27]. The relative risk of mortality compared to population mortality by age and over time after hip and vertebral fracture was derived from two Swedish studies [10, 27]. No excess mortality was assumed for NHNV-fractures.
Patients with osteoporosis have a higher degree of frailty compared to the general population, and excess mortality after a fragility fracture is not entirely attributable to the fracture event. In agreement with previous health economic studies, it was assumed that 30% of excess mortality after a fracture is associated with the fracture event [21, 28]. The increased mortality was assumed to persist for 8 years, in line with a pervious study [28].
Costs and quality of life
Cost of hip fractures in the first year was taken from two Belgian publications [29, 30]. Cost of vertebral fractures were calculated as a fraction of hip fracture cost for Belgium, according to the number of hip fracture morbidity equivalents at age of 70 [31]. These costs are conservative as they do not take into account the increased fracture costs with increasing age, as described in other publications [31]. Cost of NHNV fractures only considered the initial phase of the medical management of fractures, excluding re-hospitalisations and nursing home stays [32, 33]. Studies measuring resources consumed in the following year after the fracture [34, 35] showed an additional cost of 34–73% of the initial management cost, which was added to the initial costs of NHNV fractures. Resource utilization, the corresponding unit costs, and sources are described in Supplementary Table 2. All costs are stated in euro (€) 2022 prices. When needed, the costs were inflated to 2022 prices using Belgian Health Index, using reference year 2013 [36]. A yearly discount rate of 3% was used for costs and a rate of 1.5% for effects in line with current recommendations [37].
The impact on quality of life during the first and subsequent years after hip, vertebral, and NHNV fractures was based on data from the International Costs and Utilities Related to Osteoporotic Fractures Study (ICUROS) [4]. The multipliers (Supplementary Table 3) were used together with population tariff values for Belgium (Supplementary Table 4).
Analysis
The main outcome was the incremental cost-effectiveness ratio (ICER) representing the additional costs required to gain one additional quality-adjusted life year (QALY) with romosozumab followed by alendronate against alendronate monotherapy and against teriparatide followed by alendronate treatment.
Deterministic sensitivity analyses (DSA) were conducted to estimate the impact on the ICER of changing one parameter input at a time. Additionally, the impact of changing persistence rate, treatment efficacy, utility multiplier, and treatment start age was tested.
Two different scenarios using the FRAX® approach for estimating fracture risk were tested. In the first scenario, the T-score was set to − 2.5, while in the second scenario, the T-score was set to − 3.2, which approximately corresponds to the average T-score for postmenopausal women with a T-score of − 2.5 and below [23].
Probabilistic sensitivity analyses (PSA) were conducted by simultaneous sampling from estimated probability distributions of model parameters to obtain 1000 sets of model input estimates. A willingness-to-pay (WTP) per QALY gained threshold of €35,000 was applied.
Results
Base case
The results from the base-case analysis are presented in Table 2. A patient treated with romosozumab-to-alendronate was expected to accrue 8.11 QALYs and a cost of €49,993. The corresponding results for alendronate monotherapy were 7.99 QALYs at a cost of €47,679. In incremental terms, romosozumab-to-alendronate was associated with 0.12 additional QALYs at an additional cost of €2314 compared to alendronate monotherapy, resulting in an ICER of €19,978.
A patient treated with teriparatide-to-alendronate was expected to accrue 7.99 QALYs and a cost of €54,421. In incremental terms, romosozumab-to-alendronate was associated with additional QALYs and lower cost, showing that it was dominant compared to teriparatide-to-alendronate.
Sensitivity analyses
Deterministic sensitivity analysis (DSA)
Table 3 presents the results from the DSA, which show the sensitivity of the ICER to various factors. The utility multiplier for vertebral and NHNV fractures at second and subsequent years was found to have the greatest impact on the ICER. Additionally, lower treatment persistence with romosozumab-to-alendronate was found to decrease cost-effectiveness compared to alendronate monotherapy. Romosozumab-to-alendronate was found to be dominant, increasing QALYs and decreasing costs, when compared to teriparatide-to-alendronate in all scenarios.
For the scenarios using FRAX® as the fracture risk assessment, the ICER was higher (€40,063) compared to the base case when the T-score was set at − 2.5. This difference was expected, as the patient population in terms of fracture risk differed between these two scenarios. However, when the T-score was set to − 3.2, which is more comparable to the base-case patient population in the traditional approach (i.e., T-score > − 2.5), the ICERs were similar (€19,948 vs. €20,533).
Probabilistic sensitivity analysis (PSA)
The PSA showed that the base-case results were robust to uncertainty in the input parameters (Supplementary Table 6). A probabilistic incremental cost of €2203 and corresponding incremental QALYs of 0.12 was estimated, resulting in a probabilistic ICER of € 18,573 (base case € 19,948) for romosozumab-to-alendronate compared to alendronate monotherapy.
A probabilistic decreasing cost of − €4,275 and corresponding incremental QALYs of 0.12 was estimated, resulting in a probabilistic dominant ICER (dominant in base case) for romosozumab-to-alendronate compared to teriparatide-to-alendronate.
The resulting cost-effectiveness acceptability curves (CEAC) are depicted in Fig. 2 and the PSA scatterplot is depicted in Fig. 3. The probability that romosozumab-to-alendronate is cost-effective vs. alendronate monotherapy is 96.8%, and vs. teriparatide-to-alendronate was 100% at a WTP for a QALY threshold of €35,000.
Discussion
The aim of this study was to evaluate the cost-effectiveness of sequential romosozumab-to-alendronate therapy in comparison to alendronate monotherapy and to sequential teriparatide-to-alendronate therapy for the management of severe osteoporosis in postmenopausal women at high risk of fractures in Belgium.
The primary finding of our study indicates that sequential romosozumab-to-alendronate therapy is expected to increase QALYs and costs, compared to alendronate monotherapy. The incremental cost-effectiveness ratio stood at €19,948 per QALY in the base-case analysis. Sensitivity analyses confirmed the robustness of the results when varying several input parameters in the model. The ICER was found to be the highest (€37,287) when the persistence with romosozumab decreased by 25%, and the lowest (€13,725) when the relative hip fracture risk reduction of romosozumab was set to the upper confidence interval limit. The probability that romosozumab-to-alendronate is cost-effective vs. alendronate monotherapy is 96.8% and vs. teriparatide-to-alendronate was 100% at a WTP for a QALY threshold of €35,000.
Compared to teriparatide-to-alendronate therapy, romosozumab-to-alendronate therapy was found to be dominant in the base case, denoting an increase in QALYs and a decrease in costs. In the sensitivity analysis, romosozumab-to-alendronate remained dominant across all tested scenarios.
In Belgium, romosozumab is also recommended in patients with a recent MOF within the last 24 months and a previous vertebral fracture that could have occurred more than 24 months before treatment regardless of T-score value. However, a cost-effectiveness analysis could not be carried out for this patient group because the model and the risk equations do not allow an accurate specification of the fracture risk in these patients. Assuming that these patients have a similar fracture risk profile as the main target patient population, however, it is reasonable to assume that the cost-effectiveness would be similar as the base-case results.
To date, only a few studies assessing the cost-effectiveness of sequential osteoporosis treatments have been published. A recent systematic review identified ten such studies, among which only two evaluated sequential romosozumab treatment [38]. The first of these publications estimated the cost-effectiveness of romosozumab-to-alendronate compared to alendronate in Sweden [39]. The base case projected the cost per QALY at €33,732 for women aged 74 years with a recent MOF. The FRAX® tool was used for fracture risk estimation in this study, making the most comparable results in our study €40,063. The second study, based on a Japanese population, evaluated romosozumab-to-alendronate compared to teriparatide-to-alendronate for women with a mean age of 78 years, a T-score < − 2.5, and a previous fragility fracture [40]. The findings showed that romosozumab-to-alendronate was associated with lower costs and more QALYs compared to teriparatide-to-alendronate, which aligns with our results.
Another recent Canadian study compared romosozumab-to-alendronate with either alendronate or risedronate in postmenopausal women with a history of osteoporotic fractures and a very high risk of future fractures [41]. This study found that romosozumab-to-alendronate was dominant compared to both alendronate and risedronate. Directly comparing cost-effectiveness results between studies conducted in different countries is challenging due to varying geographies, methodological approaches, epidemiological patterns, costs, and patient population definitions. However, these published studies, along with ours, indicate that romosozumab-to-alendronate is a cost-effective treatment option for patients at a high risk of fracture compared to both alendronate and teriparatide-to-alendronate.
In this study, alendronate was selected as the primary comparator and the sequential treatment for romosozumab, given its status as the most frequently used treatment in the relevant patient population. Moreover, alendronate served as the comparator in the phase III study of romosozumab [7]. Romosozumab-to-alendronate was also compared to teriparatide-to-alendronate. Before the approval of romosozumab, teriparatide was the only approved osteoanabolic bone-forming treatment for osteoporosis in Belgium. This analysis, however, has certain limitations because the sequential effect on fracture risk after teriparatide has not been studied in a large randomized controlled clinical trial, as it was for romosozumab. The treatment effect of sequential alendronate after teriparatide needed to be derived from studies that included prior treatment.
The treatment duration of teriparatide was set to 18 months which is in line with Belgian reimbursement criteria. In some other countries, teriparatide is reimbursed for 24 months of use. For example, in cost-effectiveness analyses conducted in Japan and Canada, the teriparatide duration was 24 months. [40, 41]
Some other limitations need to be taken into account. First, real-world persistence data for romosozumab-to-alendronate is currently unavailable. In the base-case scenario, we assumed that 90% of patients would persist throughout the 12-month treatment period, in agreement with the compliance rate for the phase III clinical trial ARCH. When the persistence was changed to 80%, the ICER increased (€25,845) but did not alter the conclusions of the study. Teriparatide demonstrates a 1-year persistence rate of approximately 70% based on clinical practice data [12]. Studies suggest that less frequent administration (e.g., daily vs. weekly) correlates with better persistence [42]. Since romosozumab is designed to be administered less frequently than teriparatide (monthly vs. daily), it is plausible that patients treated with romosozumab will exhibit better persistence than those treated with teriparatide. Using a higher persistence rate for romosozumab than for teriparatide has been accepted by the Belgian HTA authority and other HTA agencies such as the National Institute of Clinical Excellence (NICE) and the Swedish Dental and Pharmaceutical Benefits Agency (TLV) due to the more convenient dosing pattern of romosozumab [11].
Another area of uncertainty involves the incidence rates for NHNV fractures used in the model. Due to the lack of robust Belgian data for these types of fractures, the incidence was inferred from Swedish data, by assuming the same ratio of clinical vertebral and NHNV fractures to hip fractures in Sweden and Belgium. As sufficient fracture incidence data is lacking in many countries, this method has become an accepted approach that has been widely applied in numerous previous studies and used by HTA authorities like the NICE [2, 3, 43].
The time-dependent increase in fracture risk after a fracture event was based on Swedish retrospective data [23], due to the absence of comparable Belgian data. Time-dependent changes in risk have been reported for Netherlands but not in the form to allow incorporation into the model [44]. While this is a limitation, it is reasonable to assume that the relative increase in fracture risk over time after a fracture is similar in Sweden and Belgium.
Overall, adverse events and serious adverse events were generally similar between treatment groups in the ARCH [7]. For this reason, no safety variables were included in the model. However, it needs to be acknowledged that romosozumab is contraindicated in patients with previous myocardial infarction or stroke [6].
The strength of this study is that it incorporates treatment sequencing and the time-dependent risk contribution from recent fractures by using a Markov microsimulation model. In addition, this study does not only evaluate the cost-effectiveness of romosozumab-to-alendronate in comparison to alendronate monotherapy which is in line with the design of the phase III clinical trial ARCH, but it also compares romosozumab-to-alendronate with teriparatide-to-alendronate. This reflects current clinical practice in Belgium, where a bone-forming therapy followed by antiresorptive therapy is recommended by the BBC treatment guidelines for the treatment of postmenopausal women with osteoporosis and a major recent osteoporotic fracture.
Conclusion
Irrespective of place in the therapy line, the results indicate that sequential treatment with romosozumab followed by alendronate is cost-effective compared to alendronate monotherapy and dominant compared to teriparatide followed by alendronate for postmenopausal women with osteoporosis at high risk of fracture in Belgium.
Data availability
Not applicable.
Code availability
Not applicable.
References
de Villiers TJ, Goldstein SR (2022) Bone health 2022: an update. Climacteric 25(1):1–3
Willers C, Norton N, Harvey NC et al (2022) Osteoporosis in Europe: a compendium of country-specific reports. Arch Osteoporos 17(1):23
Kanis JA, Norton N, Harvey NC et al (2021) SCOPE 2021: a new scorecard for osteoporosis in Europe. Arch Osteoporos 16(1):82
Kanis JA, Johansson H, Oden A et al (2018) Characteristics of recurrent fractures. Osteoporos Int 29(8):1747–1757
Sanchez-Rodriguez D, Bergmann P, Body JJ et al (2020) The Belgian Bone Club 2020 guidelines for the management of osteoporosis in postmenopausal women. Maturitas 139:69–89
(2020) EVENITY (romosozumab) summary of product characteristics. [cited 2023 13.04.2023]; Available from: https://www.ema.europa.eu/en/documents/product-information/evenity-epar-product-information_en.pdf
Saag KG, Petersen J, Brandi ML et al (2017) Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med 377(15):1417–1427
Willems D, Javaid MK, Pinedo-Villanueva R et al (2022) Importance of time point-specific indirect treatment comparisons of osteoporosis treatments: a systematic literature review and network meta-analyses. Clin Ther 44(1):81–97
Söreskog E, Borgström F, Lindberg I et al (2021) A novel economic framework to assess the cost-effectiveness of bone-forming agents in the prevention of fractures in patients with osteoporosis. Osteoporos Int 32(7):1301–1311
Jonsson B, Strom O, Eisman JA et al (2011) Cost-effectiveness of denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int 22(3):967–982
INAMI (2023) Médicaments remboursables (mise à jour: 01/08/2023). 2023–08–11]; Available from: https://webappsa.riziv-inami.fgov.be/SSPWebApplicationPublic/fr/Public/ProductSearch
Landfeldt E, Strom O, Robbins S, Borgstrom F (2012) Adherence to treatment of primary osteoporosis and its association to fractures–the Swedish Adherence Register Analysis (SARA). Osteoporos Int 23(2):433–443
Inbeeo (2020) A Delphi panel to elicit treatment pathways, persistence and adherence for osteoporosis drugs in Belgium. Internal report funded by UCB
Morley J, Moayyeri A, Ali L et al (2020) Persistence and compliance with osteoporosis therapies among postmenopausal women in the UK Clinical Practice Research Datalink. Osteoporos Int 31(3):533–545
Li L, Roddam A, Gitlin M et al (2012) Persistence with osteoporosis medications among postmenopausal women in the UK General Practice Research Database. Menopause 19(1):33–40
Hiligsmann M, Bruyère O, Roberfroid D et al (2012) Trends in hip fracture incidence and in the prescription of antiosteoporosis medications during the same time period in Belgium (2000–2007). Arthritis Care Res (Hoboken) 64(5):744–750
Kanis JA, Johnell O, Oden A et al (2002) Intervention thresholds for osteoporosis. Bone 31(1):26–31
Kanis JA, Johnell O, Oden A et al (2000) Long-term risk of osteoporotic fracture in Malmo. Osteoporos Int 11(8):669–674
Kanis JA, Oden A, Johnell O et al (2001) The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 12(5):417–427
Strom O, Borgstrom F, Kleman M et al (2010) FRAX and its applications in health economics–cost-effectiveness and intervention thresholds using bazedoxifene in a Swedish setting as an example. Bone 47(2):430–437
Strom O, Borgstrom F, Sen SS et al (2007) Cost-effectiveness of alendronate in the treatment of postmenopausal women in 9 European countries–an economic evaluation based on the fracture intervention trial. Osteoporos Int 18(8):1047–1061
Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19(4):385–397
Soreskog E, Strom O, Spangeus A et al (2020) Risk of major osteoporotic fracture after first, second and third fracture in Swedish women aged 50 years and older. Bone 134:115286
Black DM, Schwartz AV, Ensrud KE et al (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 296(24):2927–2938
Prince R, Sipos A, Hossain A et al (2005) Sustained nonvertebral fragility fracture risk reduction after discontinuation of teriparatide treatment. J Bone Miner Res 20(9):1507–1513
STATBEL (2021) National life tables, Belgium;, 1994–2021 (Office for national statistics). Central rate of mortality, females.,. Available from: https://statbel.fgov.be/en/themes/population/mortality-life-expectancy-and-causes-death/life-expectancy-and-life-tables#panel-12
Johnell O, Kanis JA, Oden A et al (2004) Mortality after osteoporotic fractures. Osteoporos Int 15(1):38–42
Kanis JA, Oden A, Johnell O et al (2003) The components of excess mortality after hip fracture. Bone 32(5):468–473
Autier P, Haentjens P, Bentin J et al (2000) Costs induced by hip fractures: a prospective controlled study in Belgium. Belgian hip fracture study group. Osteoporos Int 11(5):373–80
Reginster JY, Gillet P, Ben Sedrine W et al (1999) Direct costs of hip fractures in patients over 60 years of age in Belgium. Pharmacoeconomics 15(5):507–514
Borgström F, Zethraeus N, Johnell O et al (2006) Costs and quality of life associated with osteoporosis-related fractures in Sweden. Osteoporos Int 17(5):637–650
Gabriel SE, Tosteson AN, Leibson CL et al (2002) Direct medical costs attributable to osteoporotic fractures. Osteoporos Int 13(4):323–330
Melton LJ 3rd, Gabriel SE, Crowson CS et al (2003) Cost-equivalence of different osteoporotic fractures. Osteoporos Int 14(5):383–388
Haentjens P, Autier P, Barette M, Boonen S (2001) The economic cost of hip fractures among elderly women. A one-year, prospective, observational cohort study with matched-pair analysis. Belgian hip fracture study group. J Bone Joint Surg Am 83(4):493–500
Kanis JA, Brazier JE, Stevenson M, Calvert NW, Lloyd Jones M (2002) Treatment of established osteoporosis: a systematic review and cost-utility analysis. Health Technol Assess 6(29):1–146
STATBEL. Consumer price index and health index. Available from: https://statbel.fgov.be/en/open-data/consumer-price-index-and-health-index
Cleemput INM, Van De Sande S, Thiry N (2012) Belgian guidelines for economic evaluations and budget impact analyses: second edition. Belgian Health Care Knowledge Centre (KCE). Reports 183
Yu G, Tong S, Liu J et al (2023) A systematic review of cost-effectiveness analyses of sequential treatment for osteoporosis. Osteoporos Int 34(4):641–658
Soreskog E, Lindberg I, Kanis JA et al (2021) Cost-effectiveness of romosozumab for the treatment of postmenopausal women with severe osteoporosis at high risk of fracture in Sweden. Osteoporos Int 32(3):585–594
Hagino H, Tanaka K, Silverman S et al (2021) Cost effectiveness of romosozumab versus teriparatide for severe postmenopausal osteoporosis in Japan. Osteoporos Int 32(10):2011–2021
Goeree R, Burke N, Jobin M et al (2022) Cost-effectiveness of romosozumab for the treatment of postmenopausal women at very high risk of fracture in Canada. Arch Osteoporos 17(1):71
Karlsson L, Lundkvist J, Psachoulia E, Intorcia M, Strom O (2015) Persistence with denosumab and persistence with oral bisphosphonates for the treatment of postmenopausal osteoporosis: a retrospective, observational study, and a meta-analysis. Osteoporos Int 26(10):2401–2411
Davis Sea (2015) Bisphosphonates for preventing osteoporotic fragility fractures (including a partial update of NICE technology appraisal guidance 160 and 161). Assessment report. Table 32., National Institute for Health and Care Excellence (NICE), Editor
van Geel TA, van Helden S, Geusens PP, Winkens B, Dinant GJ (2009) Clinical subsequent fractures cluster in time after first fractures. Ann Rheum Dis 68(1):99–102
Acknowledgements
Research support for data inputs and result interpretation was provided by Agnes Degroote, MSc, and funded by UCB Pharma.
Funding
This study was funded by UCB Pharma and Amgen Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
EG is a board member of the Belgian Bone Club. EG has received consultancy fees and lecture fees from Danone, Amgen, Orifarm and UCB, unrelated to this work.MA is an employee of Quantify Research which was contracted and paid by UCB Pharma to conduct the study. FB owns stock in Quantify Research. The authors did not receive direct payment as a result of this work outside of their normal salary payments. DW and ES are employees and shareholders of UCB Pharma. JAK is a director of Osteoporosis Research Ltd that maintains FRAX®.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gielen, E., Aldvén, M., Kanis, J.A. et al. Cost-effectiveness of romosozumab for the treatment of postmenopausal women with osteoporosis at high risk of fracture in Belgium. Osteoporos Int (2024). https://doi.org/10.1007/s00198-024-07043-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00198-024-07043-2