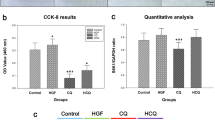
Abstract
Hepatocellular carcinoma (HCC) is an aggressive malignancy, and its effective treatment has been hampered by drug resistance. Extracellular vesicle (EV) delivery of TNF-related apoptosis-inducing ligand (TRAIL) (EV-T) was demonstrated to be superior to recombinant TRAIL (rTRAIL) for cancer treatment previously. And AZD5582, a potent antagonist of inhibitors of apoptosis proteins (IAPs) can potentiate apoptosis-based cancer therapies. However, the combination of EV-T and AZD5582 has never been examined for their possible apoptosis inducing synergism in cancers. In this study, we proposed and tested the combination of EV-T and AZD5582 as a potential novel therapy for effective treatment of HCC. Two HCC lines Huh7 and HepG2 that are both resistant to rTRAIL were examined. The results confirmed that AZD5582 and EV-T are synergistic for apoptosis induction in some cancer lines including Huh7 and HepG2 while sparing normal cells. More importantly, this study revealed that TRAIL sensitization by AZD5582 is mediated through the concomitant suppression of anti-apoptotic factors including cFLIP, MCL-1, and IAPs (XIAP, Survivin and cIAP-1). Particularly the downregulation of cFLIP and IAP's appeared to be essential and necessary for the synergism between AZD5582 and TRAIL. In vivo, we first time demonstrated that the combined therapy with low doses of AZD5582 and EV-Ts triggered drastically enhanced apoptosis leading to the complete eradication of Huh7 tumor development without any apparent adverse side effects examined. We thus have unraveled the important molecular mechanism underlying TRAIL sensitization by AZD5582, rationalizing the next development of a combination therapy with AZD5582 and EV-T for HCC treatment.
Key messages
-
It confirmed the TRAIL sensitization by AZD5582, a potent antagonist of IAPs in hepatocarcinoma.
-
It revealed that the sensitization is via the concomitant suppression of antiapoptotic factors including cFLIP, MCL-1, and IAPs.
-
The downregulation of cFLIP and IAPs like Survivin appeared to be essential and necessary for the synergism between AZD5582 and nanosomal TRAIL.
-
In vivo the combined therapy with AZD5582 and nanosomal TRAIL led to complete eradication of hepatocarcinoma tumors.
-
This study has rationalized the next development of a combination therapy with AZD5582 and nanosomal TRAIL for cancer treatment.








Similar content being viewed by others
Availability of data and materials
Not applicable.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- EV:
-
Extracellular vesicle
- EV-T:
-
TRAIL-expressing extracellular vesicle
- SMAC:
-
Second mitochondria-derived activator of caspase
- rTRAIL:
-
Recombinant TRAIL
- CCK-8:
-
Cell Counting Kit-8
- TRAIL:
-
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand
- DR:
-
Death receptor
- MSC:
-
Mesenchymal stem cell
- FADD:
-
Fas-associated with death domain protein
- DISC:
-
Death-inducing signaling complex
- MFI:
-
Median fluorescence intensity
- FBS:
-
Fetal bovine serum
- CDK:
-
Cyclin-dependent kinases
- MOI:
-
Multiplicity of infection
- Caspase:
-
Cysteinyl aspartate specific proteinase
- PBS:
-
Phosphate buffered saline
- IHC:
-
Immuno histochemistry
- H&E:
-
Hematoxylin-eosin staining
- IAPs:
-
Inhibitors of apoptosis proteins
- NTA:
-
Nanoparticle tracking analysis
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J Clin 71:209–249
Sawada Y, Yoshikawa T, Nobuoka D, Shirakawa H, Kuronuma T, Motomura Y et al (2012) Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res 18:3686–3696
Kole C, Charalampakis N, Tsakatikas S, Vailas M, Moris D, Gkotsis E et al (2020) Immunotherapy for hepatocellular carcinoma: a 2021 update. Cancers (Basel) 12
Kim DW, Talati C, Kim R (2017) Hepatocellular carcinoma (HCC): beyond sorafenib-chemotherapy. J Gastrointest Oncol 8:256–265
Giannini EG, Farinati F, Ciccarese F, Pecorelli A, Rapaccini GL, Di Marco M et al (2015) Prognosis of untreated hepatocellular carcinoma. Hepatology 61:184–190
Jiang W, Lu Z, He Y, Diasio RB (1997) Dihydropyrimidine dehydrogenase activity in hepatocellular carcinoma: implication in 5-fluorouracil-based chemotherapy. Clin Cancer Res 3:395–399
Kato A, Miyazaki M, Ambiru S, Yoshitomi H, Ito H, Nakagawa K et al (2001) Multidrug resistance gene (MDR-1) expression as a useful prognostic factor in patients with human hepatocellular carcinoma after surgical resection. J Surg Oncol 78:110–115
Straub CS (2011) Targeting IAPs as an approach to anti-cancer therapy. Curr Top Med Chem 11:291–316
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S et al (2021) Hepatocellular carcinoma Nat Rev Dis Primers 7:6
Tang W, Chen Z, Zhang W, Cheng Y, Zhang B, Wu F et al (2020) The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct Target Ther 5:87
Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A (1996) Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem 271:12687–12690
Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M et al (1999) Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 5:157–163
Micheau O, Shirley S, Dufour F (2013) Death receptors as targets in cancer. Br J Pharmacol 169:1723–1744
Thapa B, Kc R, Uludağ H (2020) TRAIL therapy and prospective developments for cancer treatment. J Control Release 326:335–349
Ciaćma K, Więckiewicz J, Kędracka-Krok S, Kurtyka M, Stec M, Siedlar M et al (2018) Secretion of tumoricidal human tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) by recombinant Lactococcus lactis: optimization of in vitro synthesis conditions. Microb Cell Fact 17:177
Wiezorek J, Holland P, Graves J (2010) Death receptor agonists as a targeted therapy for cancer. Clin Cancer Res 16:1701–1708
Wong SHM, Kong WY, Fang CM, Loh HS, Chuah LH, Abdullah S et al (2019) The TRAIL to cancer therapy: Hindrances and potential solutions. Crit Rev Oncol Hematol 143:81–94
Dimberg LY, Anderson CK, Camidge R, Behbakht K, Thorburn A, Ford HL (2013) On the TRAIL to successful cancer therapy? Predicting and counteracting resistance against TRAIL-based therapeutics. Oncogene 32:1341–1350
Wang F, Lin J, Xu R (2014) The molecular mechanisms of TRAIL resistance in cancer cells: help in designing new drugs. Curr Pharm Des 20:6714–6722
von Karstedt S, Montinaro A, Walczak H (2017) Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat Rev Cancer 17:352–366
Loebinger MR, Sage EK, Davies D, Janes SM (2010) TRAIL-expressing mesenchymal stem cells kill the putative cancer stem cell population. Br J Pharmacol 103:1692–1697
Sage EK, Kolluri KK, McNulty K, Lourenco Sda S, Kalber TL, Ordidge KL et al (2014) Systemic but not topical TRAIL-expressing mesenchymal stem cells reduce tumour growth in malignant mesothelioma. Thorax 69:638–647
Spano C, Grisendi G, Golinelli G, Rossignoli F, Prapa M, Bestagno M et al (2019) Soluble TRAIL armed human msc as gene therapy for pancreatic cancer. Sci Rep 9:1788
Yuan Z, Lourenco Sda S, Sage EK, Kolluri KK, Lowdell MW, Janes SM (2016) Cryopreservation of human mesenchymal stromal cells expressing TRAIL for human anti-cancer therapy. Cytotherapy 18:860–869
Yuan Z, Kolluri KK, Gowers KH, Janes SM (2017) TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy. J Extracell Vesicles 6:1265291
Ke C, Hou H, Li J, Su K, Huang C, Lin Y et al (2020) Extracellular vesicle delivery of TRAIL eradicates resistant tumor growth in combination with CDK inhibition by dinaciclib. Cancers (Basel) 12
Hou H, Su K, Huang C, Yuan Q, Li S, Sun J et al (2021) TRAIL-Armed ER nanosomes induce drastically enhanced apoptosis in resistant tumor in combination with the antagonist of IAPs (AZD5582). Adv Healthc Mater 10:e2100030
Yuan Z, Kolluri KK, Sage EK, Gowers KH, Janes SM (2015) Mesenchymal stromal cell delivery of full-length tumor necrosis factor-related apoptosis-inducing ligand is superior to soluble type for cancer therapy. Cytotherapy 17:885–896
Rao Q, Zuo B, Lu Z, Gao X, You A, Wu C et al (2016) Tumor-derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and humans in vitro. Hepatology 64:456–472
Hennessy EJ, Adam A, Aquila BM, Castriotta LM, Cook D, Hattersley M et al (2013) Discovery of a novel class of dimeric Smac mimetics as potent IAP antagonists resulting in a clinical candidate for the treatment of cancer (AZD5582). J Med Chem 56:9897–9919
Belhadj Z, He B, Deng H, Song S, Zhang H, Wang X et al (2020) A combined “eat me/don’t eat me” strategy based on extracellular vesicles for anticancer nanomedicine. J Extracell Vesicles 9:1806444
O’Connor L, Harris AW, Strasser A (2000) CD95 (Fas/APO-1) and p53 signal apoptosis independently in diverse cell types. Cancer Res 60:1217–1220
Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA et al (1999) Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 104:155–162
Chen J, Yu X, Huang Y (2016) Inhibitory mechanisms of glabridin on tyrosinase. Spectrochim Acta, Part A 168:111–117
Lemke J, von Karstedt S, Abd El Hay M, Conti A, Arce F, Montinaro A et al (2014) Selective CDK9 inhibition overcomes TRAIL resistance by concomitant suppression of cFlip and Mcl-1. Cell Death Differ 21:491–502
Polanski R, Vincent J, Polanska UM, Petreus T, Tang EK (2015) Caspase-8 activation by TRAIL monotherapy predicts responses to IAPi and TRAIL combination treatment in breast cancer cell lines. Cell Death Dis 6:e1893
Baglio SR, Pegtel DM, Baldini N (2012) Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol 3:359
Walker S, Busatto S, Pham A, Tian M, Suh A, Carson K et al (2019) Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 9:8001–8017
Armstrong JP, Holme MN, Stevens MM (2017) Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano 11:69–83
El-Andaloussi S, Lee Y, Lakhal-Littleton S, Li J, Seow Y, Gardiner C et al (2012) Exosome-mediated delivery of siRNA in vitro and in vivo. Nat Protoc 7:2112–2126
Hamidi H, Ivaska J (2018) Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer 18:533–548
Funding
This work was supported by the Guangdong Provincial Talented Scholar Foundation (grant number 220418137) and the National Natural Science Foundation of China (grant number 82173850).
Author information
Authors and Affiliations
Contributions
K.S., Q.Y., and H.H. contributed equally to this work. Conceptualization, K.S. Q.Y., H.H., X.P.L, and Z.Q.Y.; methodology, K.S., H.H., C.H.K., C.H.H., Y.Q.C., W.J.S., H.J.X, and Q.Y.; formal analysis, Z.Q.Y., X.P.L., C.H.K., and H.H; investigation, K.S., H.H., C.H.K., Q.Y., S.Y.L., X.Y., and J.W.S; resources, Z.Q.Y., Z.Y.D., and X.P.L.; writing-original draft preparation, K.S., Q.Y., H.H., and Z.Q.Y.; funding Acquisition, Z.Q.Y.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures and protocols for in vivo study had been approved by the Animal Ethics Committee in South China University of Technology (Approval ID: 20201210022; Date: 22 October 2020). All animal model experiments were carried out in accordance with the ethical standards outlined in the Best Practice Guidelines on Publishing Ethics.
Consent for publication:
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Su, K., Yuan, Q., Hou, H. et al. EV-T synergizes with AZD5582 to overcome TRAIL resistance through concomitant suppression of cFLIP, MCL-1, and IAPs in hepatocarcinoma. J Mol Med 100, 629–643 (2022). https://doi.org/10.1007/s00109-022-02180-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-022-02180-9