Abstract
Pseudomonas aeruginosa is an important opportunistic pathogen that has become a serious problem due to increased rates of antibiotic resistance. Due to this along with a dearth in novel antibiotic development, especially against Gram-negative pathogens, new therapeutic strategies are needed to prevent a post-antibiotic era. Here, we describe the importance of the vacJ/Mla pathway in resisting bactericidal actions of the host innate immune response. P. aeruginosa tn5 transposon mutants in genes from the VacJ/Mla pathway showed increased susceptibility to killing by the host cathelicidin antimicrobial peptide, LL-37, when compared to the wild-type parent strain. The P. aeruginosa vacJ − mutant demonstrated increased membrane permeability upon damage as well as sensitivity to killing in the presence of the detergent sodium dodecyl sulfate and the divalent cation chelator EDTA. When exposed to human whole blood and serum complement, the vacJ − mutant was killed more rapidly when compared to the wild-type parent strain and complemented mutant. Finally, in an in vivo mouse lung infection model, infection with the vacJ − mutant resulted in reduced mortality, lower bacterial burden, and reduced lung damage when compared to the wild-type strain. This study highlights the potential in therapeutically targeting the VacJ/Mla pathway in sensitizing P. aeruginosa to killing by the host innate immune response.
Key messages
• The Mla pathway regulates outer membrane dynamics in human pathogen Pseudomonas aeruginosa (PA).
• Disruption of Mla pathway gene vacJ sensitizes PA to host cathelicidin antimicrobial peptide LL-37.
• Loss of vacJ expression renders PA more sensitive to human whole blood and serum killing.
• Loss of vacJ expression reduces PA survival and virulence in a murine lung infection model.
• The Mla pathway merits exploration as a pharmacologic target to sensitize PA to host innate immunity.
Similar content being viewed by others
Introduction
Pseudomonas aeruginosa is a ubiquitous Gram-negative facultative anaerobic bacterium that inhabits both soil and aqueous environments. A leading opportunistic human pathogen, P. aeruginosa, is highly adaptable and intrinsically able to resist diverse antibiotic classes posing a tremendous challenge to medical management [1]. P. aeruginosa preferentially colonizes immunocompromised patients thereby causing many nosocomial infections including ventilator-associated pneumonia, urinary tract infections, bacteremia, and infections of surgical sites or burn wounds [1, 2]. Individuals with cystic fibrosis are at extreme risk of chronic pulmonary infection with P. aeruginosa, with 47.5% of patients being colonized [3]. P. aeruginosa can also cause community-acquired infections including keratitis, otitis externa, and skin and soft tissue infections. Therapeutic options are dwindling due to a decrease in novel antibiotic development as well as an emergence of highly multidrug-resistant P. aeruginosa strains, placing the pathogen on the Centers for Disease Control list of “serious threats” [4]. The discovery of new targets and alternate strategies for treating P. aeruginosa infections is imperative.
Gram-negative bacteria such as P. aeruginosa are protected by their outer membrane (OM), which is highly impermeable to toxic compounds such as detergents, bile salts, and antibiotics [5]. This OM is composed of an asymmetric bilayer containing lipopolysaccharide (LPS) on the outer leaflet and phospholipids (PLs) on the inner leaflet. Perturbation of the OM can lead to accumulation of PLs on the outer leaflet, which can make the bacteria vulnerable to further OM damage. The Mla system is involved in maintaining OM lipid asymmetry and is composed of seven proteins that are distributed across the cell envelope [6, 7]. VacJ (also known as MlaA) and other components of the Mla pathway have been shown to play a role in intracellular spread of Shigella flexneri [8, 9], for survival of Haemophilus influenzae during lung infections and in serum [10, 11], resistance to surfactants in Escherichia coli [6, 12, 13] and Pseudomonas putida [14], antibiotic resistance in P. aeruginosa [15, 16] and Salmonella enterica serovar Typhimurium [17], and has a possible role in the creation of outer membrane vesicles [18]. VacJ has also been explored as a possible vaccine target in Pasteurella multocida [19]. Genes encoding components of the Mla pathway, including VacJ, are upregulated in response to colistin, and mutations in the pathway have been observed in colistin-resistant mutants [20,21,22].
Among the critical frontline effectors of host innate immune defense are endogenous cationic antimicrobial peptides (AMPs), which are produced by epithelial cells and circulating or tissue-resident leukocytes, and are known to provide protection against invasive bacterial infections [23, 24]. Cathelicidins are a family of mammalian AMPs with broad-spectrum antimicrobial activity against Gram-negative and Gram-positive bacteria, as well as certain fungi, viruses, and protozoan parasites [25]. The human cathelicidin AMP, LL-37, is an alpha-helical, amphipathic, and anionic peptide that integrates and destabilizes bacterial cell membranes causing bacterial death [26]. LL-37 also has important immunomodulatory roles in the proper regulation of cytokine release, chemotaxis, and antigen presentation (reviewed in [27]). Animals deficient in cathelicidin are hyper-susceptible to bacterial infection in multiple organs including the skin [28], lung [29, 30], gut [31, 32], brain [33], kidney [34], and eye [35]. A bacterial pathogen producing significant clinical disease in the human host must maintain some level of functional resistance to endogenous AMPs including cathelicidin in order for infection to persist [36, 37].
In this study, we explored for the first time the role(s) of the Mla pathway in P. aeruginosa resistance to host innate immune defenses and animal virulence. Though failing to show a growth defect in standard bacteriological and tissue culture media conditions, P. aeruginosa Mla pathway mutants showed increased susceptibility to human cathelicidin antimicrobial peptide LL-37 associated with increased membrane permeability. Compared to wild-type parent strain, an isogenic P. aeruginosa vacJ − mutant showed increased susceptibility to LL-37 and the cationic peptide antibiotics colistin and polymyxin B, along with decreased survival in human whole blood and serum containing active complement; wild-type resistance to these factors was restored by complementation of the vacJ − mutant with the wild-type gene with a transposon insertion. The P. aeruginosa vacJ − mutant showed decreased virulence in a murine in vivo pneumonia model, as measured by lower bacterial burden in the lungs and blood, diminished lung injury and inflammation, and reduced mortality. Together, our results show the importance of the Mla pathway in P. aeruginosa resistance to the bactericidal activities of the innate immune system.
Materials and methods
Bacterial strains and reagents
P. aeruginosa PAO1 [38] along with corresponding transposon Tn5 IS50L mutants [39] disrupting the genes encoding mlaA (vacJ) and mlaC-F were generously provided by Professor Colin Manoil (University of Washington, Seattle, WA). Strains were propagated in Luria broth (LB) medium (Hardy Diagnostics) or on LB agar (LA) plates at 37 °C. For infection studies, bacteria were grown to mid-log phase (OD600 nm = 0.4) before diluting to experimental inoculum. Complementation was performed as previously described [40]. Briefly, P. aeruginosa vacJ gene and its upstream region were cloned into pUC18T-mini-Tn7T-hph using primers PA2800-miniTn7t-F (TGCTGCAAAGCCTGCACGGATCCACTAGTGAGCTCATGC), PA2800-miniTn7t-R (GAGGTGGAGGACGACTTCTAACCAGATAAGTGAAATCTAGTTCAAAAC), miniTn7t-PA2800-F (GCATGAGCTCACTAGTGGATCCGTGCAGGCTTTGCAGCA), and miniTn7T-PA2800-R (GTTTGGAACTAGATTTCACTTATCTGGTTAGAAGTCGTCCTCCACCTC). PA2800-miniTn7t-F and -R primers were used to amplify the pUC18T-mini-Tn7T-hph PCR product with portions of the vacJ gene, while primers miniTn7t-F and -R were used to amplify the vacJ gene with a portion of pUC18T-mini-Tn7T-hph. PCR products were combined 1:1 and electroporated into E. coli TOP10 cells, and transformants selected on LB agar plates containing 100 μg/mL Hygromycin B (Life Technologies). qPCR of wild-type, vacJ mutant, and complemented strains was performed on RNA isolated using RNeasy mini kit (Qiagen) and following primers: PAO1_PA2800_qPCR_F (GCGTACGTCGTCCATGTAGT), PAO1_PA2800_qPCR_R (CAGGCCAAGTTCCACAATGC), PAO1_16s_F (CCCAACATCTCACGACACGA), and PAO1_16s_R (ACGCGAAGAACCTTACCTGG).
Antibiotics and antimicrobial peptides
For in vitro studies, colistin sulfate (Sigma-Aldrich) stock solutions were prepared in molecular biology grade water at 10,000 mg/L and frozen at − 80 °C. LL-37 and TAMRA fluorescently tagged LL-37 were purchased from the American Peptide Company; stock solutions were prepared in molecular biology grade water at 640 and 320 μM, respectively, and stored at − 80 °C. LL-37 minimum inhibitory concentration (MIC) assays were performed in RPMI containing 5% LB with 5 × 105 cfu/mL in 96-well round bottom plates (Corning) while all others were performed in cation-adjusted Mueller Hinton broth (Hardy Diagnostics). All MICs were performed by serial 2-fold dilutions, repeated 3 times.
NPN bacterial outer membrane permeability assay
Assay was performed as previously described [41]. Briefly, overnight cultures of P. aeruginosa were grown in LB at 37 °C while shaking, washed twice with PBS via centrifugation at 3220×g at room temperature, and resuspended to OD600 nm of 0.40 in 6 mL of RPMI + 5% LB. LL-37 was added to a final concentration of 4 μM to a subset of the bacterial cultures. Cultures were shaken at 37 °C for 1 h, spun at 3000×g at room temperature for 5 min, and resuspended in 0.5 mL of 10 mM Tris buffer pH 8.0. The concentrated 0.5 mL cultures were used to prepare 1 mL bacterial stocks at OD600 nm of 0.40 in 10 mM Tris. Assays were conducted in a final volume of 200 μL in triplicate in 96-well flat bottom plates (Costar). Four conditions were tested: (1) 100 μL of bacterial stock + 50 μL of NPN (40 μM final) + 50 μL of 10 mM Tris, (2) 100 μL of bacterial stock + 50 μL of NPN + 50 μL of EDTA (10 mM final), (3) 100 μL of bacterial stock + 50 μL of EDTA + 50 μL of 10 mM Tris, and (4) 100 μL of 10 mM Tris + 50 μL of NPN + 50 μL of EDTA. As soon as all of the components were added and mixed, plates were immediately read in a fluorescent plate reader: excitation 250 nm and emission 420 nm. The NPN fluorescence signal from conditions (3) and (4), background, was subtracted from the signals measured from conditions (1) and (2). The NPN intensity from condition (1) bacteria + NPN was divided by the NPN signal measured from condition (2) bacteria + NPN + EDTA to obtain the percentage of permeability recorded in the presence of 10 mM EDTA which permeabilizes the outer membrane of Gram-negative bacteria.
Whole blood and serum killing
Heparinized blood was collected from healthy adult volunteers and placed in siliconized tubes. 1 x 106 cfu of P. aeruginosa in RPMI was added to whole blood to make final concentration 90% whole blood in 100 μl volume. Samples were taken at time 0, 15, 30, 45, and 60 min, serially diluted, and plated on LB agar plates for cfu enumeration. Serum was collected from healthy volunteers by collecting blood in serum separating tubes (BD) which were spun at 1300×g for 10 min to separate serum from erythrocytes and other cells. Collected serum was pooled and split; half was heat-inactivated at 56 °C for 30 min while the other half was stored at room temperature for 30 min. Serum was partitioned into wells of a 96-well round bottom plate and 1 × 106 cfu of P. aeruginosa wild-type, vacJ −, or complemented mutant in 10 μl RPMI was added to a final serum concentration of 80% in 100 μl volume. Samples were collected at 60, 120, and 180 min, serially diluted, and plated on LB agar for enumeration. Experiments were performed in triplicate.
Murine pneumonia model
Eight-week-old female C57BL/6J mice (Jackson Labs) were used. P. aeruginosa cultures were grown overnight in LB at 37 °C with shaking, washed twice with PBS, diluted 1:50 in fresh LB, and grown to mid-log phase. Bacteria were washed twice with PBS via centrifugation at 3220×g at room temperature and concentrated in PBS to yield the appropriate inoculum. For histopathology, lung bacterial load, and blood bacterial load, 4 × 106 cfu per 30 μL was used to infect each animal (WT infection n = 10; vacJ − infection n = 10). Mice were anesthetized with 100 mg/kg ketamine + 10 mg/kg xylazine. Once sedated, the vocal cords were visualized using an operating otoscope (Welch Allyn) and 30 μL of bacteria or PBS instilled into the trachea during inspiration using a plastic gel loading pipette tip. Mice were placed on a warmed pad for recovery. After 24 h, mice were sacrificed with CO2 and the lungs were collected for bacterial counts and histopathological analysis. To enumerate bacteria remaining in the lungs, the organs were placed in a 2 mL sterile micro tube (Sarstedt) containing 1 mL of PBS and 1 mm silica beads (Biospec). The lungs were homogenized by shaking at 6000 rpm for 30 s (× 3) using a MagNA Lyser (Roche), with the specimens placed on ice as harvested. Aliquots from each tube were serially diluted for enumeration on LB agar plates. For blood counts, mice were infected as described and sacrificed 24 h post-infection and blood obtained by cardiac puncture. For the P. aeruginosa survival experiment, 8.5 × 106 cfu was given in 30 μL of PBS (WT infection n = 15; vacJ − infection n = 15); animals were monitored every 12 h for 7 days. All animal studies were performed under protocols approved by the UCSD Institutional Animal Use and Care Committee with national and local guidelines in place to maximize humane animal treatment.
Histopathologic scoring
A veterinary pathologist (IC), blinded to treatment, scored the lung lesions from five mice infected with WT P. aeruginosa and five mice infected with the vacJ − strain based on severity of bacterial load, neutrophilic inflammatory cell infiltration, tissue damage/necrosis, and percent lung tissue involvement. The lungs were then ranked 1–10 from most to least severe based on a composite of lesion severity and distribution. Lesion ranks from the two groups (WT and vacJ −) were compared by Mann-Whitney test (two-tailed analysis).
Imaging
Tissue sections were imaged on a Nikon E800M microscope using a Nikon DSRi2 camera and Nikon Elements D Version 4 software. Each section was imaged using the same white balance and shading correction settings. Whole image sharpness, contrast, and brightness were uniformly adjusted with Photoshop Elements version 11 software.
Results
Loss of function P. aeruginosa mla mutants are hypersusceptible to antimicrobial peptides
In recent years, the roles of the Mla pathway to resist stresses to the OM have been described; it is involved in resistance to hydrophobic compounds [14, 42], ethanol [14, 42], serum [11], and certain antibiotics [15]. To determine if P. aeruginosa mutants containing transposon insertions in genes encoding Mla pathway proteins (Fig. 1a) were susceptible to outer membrane attack by the human cathelicidin antimicrobial peptide LL-37, a minimum inhibitory concentration (MIC) assay was performed. Wild-type P. aeruginosa consistently resisted the bactericidal action of LL-37 up to a concentration of 16 μM while all mla transposon insertion mutants revealed a consistent 2-fold reduction in MIC at 8 μM (Fig. 1 c). We next complemented one of the mla mutants, vacJ − with a mini-Tn7t transposon containing the P. aeruginosa PAO1 vacJ gene. Complementation restored over half (58%) of transcript levels back to the mutant (Fig. 1b). A wild-type LL-37 resistance phenotype was restored to the complemented vacJ − mutant (Fig. 1 c). Challenging the P. aeruginosa vacJ − mutant with the murine cathelicidin related antimicrobial peptide (mCRAMP) yielded a similar 2-fold increased sensitivity compared to the wild-type and the vacJ − complemented mutant strains (Table 1). Acceleration in LL-37 killing kinetics of vacJ − mutant lacking a functional Mla system was observed in time course assays at 16 μM (Fig. 1d) and 8 μM (Fig. 1e) LL-37 compared to both the wild-type and the complemented strains.
P. aeruginosa mla deficient mutants demonstrate increased sensitivity to antimicrobial peptide LL-37. Position of transposons for all mla deficient strains are denoted by black arrows (a). qPCR of transcript levels for vacJ, normalized to 16S rRNA and Wt levels (b). LL-37 MIC for P. aeruginosa wild-type, mla deficient mutants, and vacJ − complemented strains. MIC results were performed 3 times (c). LL-37 killing kinetics of P. aeruginosa WT, vacJ −, and vacJ − complemented strains at 16 μM (d) and 8 μM (e). **P < 0.01 t test
The related cationic peptide antibiotics colistin and polymyxin B are increasingly being used as last-line agents for treatment of patients with highly multidrug-resistant strains of P. aeruginosa [43, 44]. Since these agents have similar charge characteristics and mechanisms of action to the host defense peptide LL-37, we examined the effect of the vacJ inactivation on sensitivity to these therapeutic agents. MIC testing revealed a 2-fold increase in susceptibility of the vacJ − mutant P. aeruginosa to both polymyxin B and colistin, which was restored to wild-type resistance levels upon genetic complementation (Table 1). Sensitivity to colistin has been observed before in the mlaE mutant of P. aeruginosa showing that this is conserved [16]. These results do not match previously reported MIC values for P. aeruginosa vacJ mutant, but this could be due to the use of different media in antibiotic susceptibility tests than a more standard methods [15]. These results show that the Mla pathway is needed in order to effectively resist the bactericidal effects of antimicrobial peptides that target the outer membrane.
P. aeruginosa mla-deficient mutants are more susceptible to cell wall stress
To ensure that cationic peptide hypersusceptibility of the vacJ − mutant did not represent a general growth defect, we confirmed equal logarithmic growth properties throughout 24-h incubation in standard bacteriologic media compared to the WT parent strain (Fig. 2a). Bacteria grown in RPMI supplemented with 5% LB also demonstrated no difference in growth rates between the wild-type, vacJ − mutant, and complemented strain in a 24-h period, though they reached a lower optical density compared to when grown in LB. To probe further into OM barrier function, we grew P. aeruginosa wild-type, vacJ − mutant, and complemented strains on agar media containing 0.5% sodium dodecyl sulfate (SDS) with varying amounts of the divalent cation chelator ethylenediaminetetraacetic acid (EDTA), known to destabilize LPS bonds and increase OM permeability. We saw an increase in SDS-EDTA sensitivity of the P. aeruginosa vacJ − mutant compared to the wild-type and complemented strains with increasing EDTA concentrations (Fig. 2b). To quantify the degree of permeability engendered, we performed an assay using the small molecule n-phenyl-1-napthylamine (NPN) which fluoresces only when it is in a hydrophobic environment, in this case the inner membrane of Gram-negative bacteria, reflecting leakiness of the OM to allow small molecules to passively cross [45]. No significant difference in NPN fluorescence was observed between P. aeruginosa wild-type and vacJ − mutant at baseline (Fig. 2c), but upon exposure to 4 μM LL-37, a 2-fold increase in permeability was seen in the vacJ − mutant compared to wild-type. The complemented strain demonstrated reduced permeability to NPN at baseline and upon LL-37 treatment (Fig. 2c). These results are consistent with a mechanism in which sensitivity to antimicrobial peptides can be attributed to an impaired OM repair in the Mla pathway mutant.
P. aeruginosa mla mutants are susceptible to OM stress with no defects in growth. Growth curves of mla mutants in Luria broth (LB) (a). Serial culture dilutions spotted on to Luria broth agar (LA), LA + 0.5% SDS and 0.25 mM EDTA, and LA + 0.5% SDS and 0.5 mM EDTA (b). Representative graph of NPN fluorescent assay measuring outer membrane permeability in either RPMI + 5% LB or RPMI + 5% LB containing 4 μM LL-37 (c). **P < 0.01, ***P < 0.001 two-way ANOVA
The P. aeruginosa vacJ − mutant is susceptible to whole blood or serum killing
To further examine the P. aeruginosa vacJ − mutant’s ability to withstand outer membrane attack generated in the context of the innate immune system, we used freshly isolated human whole blood that contains several OM active components (complement, platelet, and neutrophil-derived antimicrobial peptides and proteases, antibodies, etc.). Within 1 h of incubation in human whole blood, we observed a log-fold increase in killing of the vacJ − mutant compared to P. aeruginosa wild-type strain (Fig. 3a); complementation of the mutant with the vacJ gene restored blood survival back to wild-type levels. Likewise, when placed in 80% human serum for 1 h, the P. aeruginosa vacJ − mutant exhibited a nearly 2-log-fold reduction in survival compared to wild-type and complemented mutant strains (Fig. 3b). When the serum was heat-inactivated to degrade active complement, the survival differences between wild-type, vacJ − mutant, and complemented mutant strain were lost. This finding illustrates the importance of having an intact Mla pathway to withstand serum- and complement-dependent killing in blood, consistent with experiments performed on the pathway in the Gram-negative coccobacillus Haemophilus influenzae [11].
Ex vivo survival of P. aeruginosa mla defective mutant vacJ −. 2.5 × 106 bacterial cfu in RPMI was placed in heparinized human whole blood to make the final concentration 90% blood in a final volume of 200 μL (a). 1 × 106 bacterial cfu in RPMI was placed in either serum (S) or heat-inactivated serum (HIS) so the final concentration was 80% serum in a final volume of 200 μL (b). *P < 0.05, **P < 0.01, ***P < 0.001 two-way ANOVA
The P. aeruginosa Mla pathway is essential for full virulence in murine pneumonia model
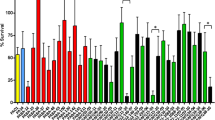
To determine the essentiality of the Mla pathway during P. aeruginosa lung infections, we used a murine pneumonia model in which 8.5 × 106 cfu of either P. aeruginosa wild-type or vacJ − mutant were administered intratracheally to 8-week-old female C57BL/6 mice. Mouse survival, bacterial load, bacteremia, and histopathology of the lungs of infected mice were assessed. P. aeruginosa wild-type infection led to 100% mortality within 3 days while the vacJ −-infected mice had 40% survival at the same time point and a final survival of 27% at the end of the 7-day experiment (P < 0.05) (Fig. 4a). In a separate cohort of mice, the lungs were harvested and homogenized to determine the bacterial burden 24 h post-infection (hpi). A 2-fold reduction in vacJ − mutant bacterial recovery compared to a wild-type infection was found (p < 0.05) (Fig. 4b). At this time point, we also observed a 3-fold reduction in bacterial cfu in the blood of mice infected with the P. aeruginosa vacJ − mutant compared to wild-type; 4 of the 6 vacJ −-infected mice had bacterial counts below 1 × 106 cfu/mL while 5 of 6 wild-type infected mice had bacterial counts greater than 1 × 106 cfu/mL (P < 0.05) (Fig. 4c). The lungs from five mice infected with either wild-type or vacJ − P. aeruginosa were examined by histology (Table 2 and Fig. 4d). Lesions were significantly more severe and widely disseminated in the wild-type infected group (p = 0.036). Four of the five mice in the wild-type group had moderate to severe lesions affecting approximately 30–50% of the lung tissue. Notably, large numbers of rod-shaped bacteria coated the alveolar walls and occasionally formed aggregates with degenerate neutrophils within the airways. Alveolar inflammation was mild to moderate and primarily neutrophilic in nature. Multiple areas of mild to moderate hemorrhage and small foci of tissue necrosis were present in severely affected animals (Wt mouse 4 and mouse 2). The remaining mouse (Wt mouse 3) had lesions more like those of the vacJ − group. Compared to wild-type infected mice, markedly fewer bacteria were seen in the lungs from mice in the vacJ − group. However, the degree of neutrophilic inflammation was generally comparable. Apart from vacJ − mouse 1, the distribution of the inflammatory lesions in the vacJ − group was less disseminated often forming several moderately well-contained foci. Hemorrhage in the vacJ − group was minimal, and foci of necrosis were infrequent. Mice in both groups exhibited moderate to severe interstitial pulmonary edema. One PBS-control mouse and one uninfected mouse were also examined. No lesions were present in the lungs from the uninfected mouse and lesions in the PBS-control mouse were minimal comprising mild alveolar infiltrates of neutrophils and macrophages in a single lung lobe. These findings demonstrate enhanced pulmonary innate immune clearance of the P. aeruginosa vacJ − mutant in concert with decreased propensity to bloodstream dissemination.
Effect of vacJ − mutation on P. aeruginosa virulence in murine pneumonia model. Mice (n = 15 per group) were infected with P. aeruginosa wild-type or vacJ − (8.5 × 106 cfu/30 μL PBS) intratracheally and observed for survival over a period of 7 days; *P < 0.05 log-rank (Mantel-Cox) test (a). Mice (n = 8 per group) were infected with P. aeruginosa wild-type or vacJ − (4 × 106 cfu/30 μL) intratracheally and sacrificed 24 h post-infection. The lungs were harvested (b) and blood collected (c) for bacterial enumeration. *P < 0.05 two-tailed Mann-Whitney test. d Top panels (scale bar = 1 mm). Left: Wt infected mouse 4, severity rank 1. Middle: vacJ − infected mouse 2, severity rank 5. Right: vacJ − infected mouse 1, severity rank 10. Alveoli (arrowheads) contain bacteria, inflammatory exudate, and hemorrhage. The interstitium is markedly expanded by edema (asterisks). Bottom panels (scale bar = 50 μm). Left: Wt infected mouse 4, severity rank 1. The alveolar walls are coated by rod-shaped bacteria (arrowheads) and multifocal hemorrhage is present (asterisks). Middle: vacJ − infected mouse 2, severity rank 5. The pulmonary architecture is disrupted by a rare focus of necrosis (outlined by arrowheads) and alveoli are occasionally filled with neutrophils. Right: vacJ − infected mouse 1, severity rank 10. Small aggregates of (arrowhead) and scattered individual neutrophils (arrow) are present in alveoli
Discussion
Nosocomial bacterial infections have become an ever-increasing public health concern, with 650,000 individuals experiencing health care-associated infections per year, at least 20% of which are characterized by antibiotic resistance [46]. Sustained selective pressure caused by the constant use of antibiotics has allowed the rise of a select group of pathogens that are commonly isolated with multidrug-resistant profiles [47]. The most challenging threats in the so-called “ESKAPE group” of pathogens are the Gram-negatives due to the dearth of novel antibiotic candidates in the pipeline to treat these infections [48]. Among Gram-negative bacterial threats, Pseudomonas is of particular concern due to the ubiquitous nature of the pathogen and its intrinsic ability to resist many antibiotic classes and mechanisms of action. P. aeruginosa is a recurring problem in older cystic fibrosis (CF) patients [3], and increasing rates of P. aeruginosa antibiotic resistance have been recently observed in isolates from central line-associated bloodstream infection as well as ventilator-associated pneumonia in the intensive care unit [46].
The Mla pathway plays a critical role in maintaining OM integrity in different Gram-negative infections but has not been studied in great detail in the context of infection and innate immune resistance among the leading antibiotic-resistant human pathogens. Here, we report that P. aeruginosa deficient in the Mla pathway and the gene vacJ in particular, though exhibiting normal growth characteristics in vitro, demonstrate increased sensitivity to endogenous and pharmaceutical cationic antimicrobial peptides, serum, and whole blood. A vacJ − mutant also showed decreased virulence in a murine model of P. aeruginosa lung infection, with reduced bacterial burden and increased survival compared to wild-type P. aeruginosa-infected mice. Much like previous results seen in Gram-negatives with Mla pathway mutations, we see that P. aeruginosa has increased susceptibility to outer membrane damage. Across murine models of infection, this susceptibility to outer membrane damage has translated to reduced virulence across a variety of Gram-negative pathogens such as Haemophilus and P. aeruginosa [16, 49]. In the Drosophila feeding assay used to probe virulence, P. aeruginosa vacJ mutant surprisingly caused increased mortality compared to wild-type and complemented strains, suggesting that there are different mechanisms at play in the model that do not translate into the murine infection models [15].
With the rapid rate of emerging Gram-negative bacterial resistance to modern antibiotics, it is critical to continue to explore alternative strategies and drug targets to combat these pathogens. Molecules designed to disrupt Gram-negative OM stability are being investigated, and show great promise as a therapeutic strategy [50]. In particular, targeting the Mla pathway could be an interesting pharmacological target as it is conserved among all Gram-negative bacteria [18] and therefore offers the potential for broad-spectrum activity and clinical utility. Our research suggests that inhibitors of the Mla pathway in P. aeruginosa would not be directly bactericidal, but would rather sensitize Gram-negative bacteria to killing by the host’s endogenous defenses (e.g., antimicrobial peptides and serum complement), potentially limiting collateral damage to the host microbiome that can be seen with repeated broad-spectrum antibiotic administration. The Mla system has also been reported to be important in maintaining colistin resistance in A. baumannii [20, 21]; inhibitors may be able to reverse colistin resistance and render them susceptible to a last line of defense antibiotic used for Gram-negative infections.
References
Driscoll JA, Brody SL, Kollef MH (2007) The epidemiology, pathogenesis and treatment of Pseudomonas Aeruginosa infections. Drugs 67:351–368
Gellatly SL, Hancock RE (2013) Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis 67:159–173
Foundation CF (2016) Cystic Fibsrosis Foundation Patient Registry, 2015 Annual Daa Report. In: Marshall BC (ed) Cystic Fibrosis Foundation, pp. 77
CDC (2013) Antibiotic Resistance Threats in the United States, 2013
Henderson JC, Zimmerman SM, Crofts AA, Boll JM, Kuhns LG, Herrera CM, Trent MS (2016) The power of asymmetry: architecture and assembly of the gram-negative outer membrane lipid bilayer. Annu Rev Microbiol. https://doi.org/10.1146/annurev-micro-102215-095308
Malinverni JC, Silhavy TJ (2009) An ABC transport system that maintains lipid asymmetry in the gram-negative outer membrane. Proc Natl Acad Sci U S A 106:8009–8014
Chong ZS, Woo WF, Chng SS (2015) Osmoporin OmpC forms a complex with MlaA to maintain outer membrane lipid asymmetry in Escherichia coli. Mol Microbiol 98:1133–1146
Suzuki T, Murai T, Fukuda I, Tobe T, Yoshikawa M, Sasakawa C (1994) Identification and characterization of a chromosomal virulence gene, vacJ, required for intercellular spreading of Shigella flexneri. Mol Microbiol 11:31–41
Carpenter CD, Cooley BJ, Needham BD, Fisher CR, Trent MS, Gordon V, Payne SM (2014) The Vps/VacJ ABC transporter is required for intercellular spread of Shigella flexneri. Infect Immun 82:660–669
Gawronski JD, Wong SM, Giannoukos G, Ward DV, Akerley BJ (2009) Tracking insertion mutants within libraries by deep sequencing and a genome-wide screen for Haemophilus genes required in the lung. Proc Natl Acad Sci U S A 106:16422–16427
Nakamura S, Shchepetov M, Dalia AB, Clark SE, Murphy TF, Sethi S, Gilsdorf JR, Smith AL, Weiser JN (2011) Molecular basis of increased serum resistance among pulmonary isolates of non-typeable Haemophilus influenzae. PLoS Pathog 7:e1001247
Han X, Dorsey-Oresto A, Malik M, Wang JY, Drlica K, Zhao X, Lu T (2010) Escherichia coli genes that reduce the lethal effects of stress. BMC Microbiol 10:35
Nakata K, Koh MM, Tsuchido T, Matsumura Y (2010) All genomic mutations in the antimicrobial surfactant-resistant mutant, Escherichia coli OW66, are involved in cell resistance to surfactant. Appl Microbiol Biotechnol 87:1895–1905
Roma-Rodrigues C, Santos PM, Benndorf D, Rapp E, Sa-Correia I (2010) Response of Pseudomonas putida KT2440 to phenol at the level of membrane proteome. J Proteome 73:1461–1478
Shen L, Gao X, Wei J, Chen L, Zhao X, Li B, Duan K (2012) PA2800 plays an important role in both antibiotic susceptibility and virulence in Pseudomonas aeruginosa. Curr Microbiol 65:601–609
McDaniel C, Su S, Panmanee W, Lau GW, Browne T, Cox K, Paul AT, Ko SH, Mortensen JE, Lam JS et al (2016) A putative ABC transporter permease is necessary for resistance to acidified nitrite and EDTA in Pseudomonas Aeruginosa under aerobic and anaerobic planktonic and biofilm conditions. Front Microbiol 7:291
Hu WS, Chen HW, Zhang RY, Huang CY, Shen CF (2011) The expression levels of outer membrane proteins STM1530 and OmpD, which are influenced by the CpxAR and BaeSR two-component systems, play important roles in the ceftriaxone resistance of Salmonella enterica serovar Typhimurium. Antimicrob Agents Chemother 55:3829–3837
Roier S, Zingl FG, Cakar F, Durakovic S, Kohl P, Eichmann TO, Klug L, Gadermaier B, Weinzerl K, Prassl R et al (2016) A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat Commun 7:10515
Shivachandra SB, Kumar A, Yogisharadhya R, Viswas KN (2014) Immunogenicity of highly conserved recombinant VacJ outer membrane lipoprotein of Pasteurella multocida. Vaccine 32:290–296
Henry R, Vithanage N, Harrison P, Seemann T, Coutts S, Moffatt JH, Nation RL, Li J, Harper M, Adler B et al (2012) Colistin-resistant, lipopolysaccharide-deficient Acinetobacter baumannii responds to lipopolysaccharide loss through increased expression of genes involved in the synthesis and transport of lipoproteins, phospholipids, and poly-beta-1,6-N-acetylglucosamine. Antimicrob Agents Chemother 56:59–69
Thi Khanh Nhu N, Riordan DW, Do Hoang Nhu T, Thanh DP, Thwaites G, Huong Lan NP, Wren BW, Baker S, Stabler RA (2016) The induction and identification of novel Colistin resistance mutations in Acinetobacter baumannii and their implications. Sci Rep 6:28291
Henry R, Crane B, Powell D, Deveson Lucas D, Li Z, Aranda J, Harrison P, Nation RL, Adler B, Harper M et al (2015) The transcriptomic response of Acinetobacter baumannii to colistin and doripenem alone and in combination in an in vitro pharmacokinetics/pharmacodynamics model. J Antimicrob Chemother 70:1303–1313
Gallo RL, Hooper LV (2012) Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol 12:503–516
Pasupuleti M, Schmidtchen A, Malmsten M (2012) Antimicrobial peptides: key components of the innate immune system. Crit Rev Biotechnol 32:143–171
Tomasinsig L, Zanetti M (2005) The cathelicidins--structure, function and evolution. Curr Protein Pept Sci 6:23–34
Sochacki KA, Barns KJ, Bucki R, Weisshaar JC (2011) Real-time attack on single Escherichia coli cells by the human antimicrobial peptide LL-37. Proc Natl Acad Sci U S A 108:E77–E81
Lai Y, Gallo RL (2009) AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol 30:131–141
Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL (2001) Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414:454–457
Kovach MA, Ballinger MN, Newstead MW, Zeng X, Bhan U, Yu FS, Moore BB, Gallo RL, Standiford TJ (2012) Cathelicidin-related antimicrobial peptide is required for effective lung mucosal immunity in Gram-negative bacterial pneumonia. J Immunol 189:304–311
Beaumont PE, McHugh B, Gwyer Findlay E, Mackellar A, Mackenzie KJ, Gallo RL, Govan JR, Simpson AJ, Davidson DJ (2014) Cathelicidin host defence peptide augments clearance of pulmonary Pseudomonas aeruginosa infection by its influence on neutrophil function in vivo. PLoS One 9:e99029
Iimura M, Gallo RL, Hase K, Miyamoto Y, Eckmann L, Kagnoff MF (2005) Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J Immunol 174:4901–4907
Hase K, Murakami M, Iimura M, Cole SP, Horibe Y, Ohtake T, Obonyo M, Gallo RL, Eckmann L, Kagnoff MF (2003) Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology 125:1613–1625
Bergman P, Johansson L, Wan H, Jones A, Gallo RL, Gudmundsson GH, Hokfelt T, Jonsson AB, Agerberth B (2006) Induction of the antimicrobial peptide CRAMP in the blood-brain barrier and meninges after meningococcal infection. Infect Immun 74:6982–6991
Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka L, Ehren I, Hokfelt T, Gudmundsson GH, Gallo RL, Agerberth B et al (2006) The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med 12:636–641
Huang LC, Reins RY, Gallo RL, McDermott AM (2007) Cathelicidin-deficient (Cnlp −/− ) mice show increased susceptibility to Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci 48:4498–4508
Nizet V (2006) Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr Issues Mol Biol 8:11–26
Strempel N, Neidig A, Nusser M, Geffers R, Vieillard J, Lesouhaitier O, Brenner-Weiss G, Overhage J (2013) Human host defense peptide LL-37 stimulates virulence factor production and adaptive resistance in Pseudomonas aeruginosa. PLoS One 8:e82240
Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M et al (2000) Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964
Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, Ernst S, Will O, Kaul R, Raymond C, Levy R et al (2003) Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 100:14339–14344
Choi KH, Schweizer HP (2006) Mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa. Nat Protoc 1:153–161
Lin L, Nonejuie P, Munguia J, Hollands A, Olson J, Dam Q, Kumaraswamy M, Rivera H Jr, Corriden R, Rohde M et al (2015) Azithromycin synergizes with cationic antimicrobial peptides to exert bactericidal and therapeutic activity against highly multidrug-resistant Gram-negative bacterial pathogens. EBioMedicine 2:690–698
Nicolaou SA, Gaida SM, Papoutsakis ET (2012) Exploring the combinatorial genomic space in Escherichia coli for ethanol tolerance. Biotechnol J 7:1337–1345
Martis N, Leroy S, Blanc V (2014) Colistin in multi-drug resistant Pseudomonas aeruginosa blood-stream infections: a narrative review for the clinician. J Inf Secur 69:1–12
Rigatto MH, Vieira FJ, Antochevis LC, Behle TF, Lopes NT, Zavascki AP (2015) Polymyxin B in combination with antimicrobials lacking in vitro activity versus Polymyxin B in monotherapy in critically ill patients with Acinetobacter baumannii or Pseudomonas aeruginosa infections. Antimicrob Agents Chemother 59:6575–6580
Helander IM, Mattila-Sandholm T (2000) Fluorometric assessment of gram-negative bacterial permeabilization. J Appl Microbiol 88:213–219
MacVane SH (2016) Antimicrobial resistance in the intensive care unit: a focus on gram-negative bacterial infections. J Intensive Care Med. https://doi.org/10.1177/0885066615619895
Khan SN, Khan AU (2016) Breaking the spell: combating multidrug resistant 'Superbugs'. Front Microbiol 7:174
Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J (2009) Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12
Zhao L, Gao X, Liu C, Lv X, Jiang N, Zheng S (2017) Deletion of the vacJ gene affects the biology and virulence in Haemophilus parasuis serovar 5. Gene 603:42–53
Titecat M, Liang X, Lee CJ, Charlet A, Hocquet D, Lambert T, Pages JM, Courcol R, Sebbane F, Toone EJ et al (2016) High susceptibility of MDR and XDR Gram-negative pathogens to biphenyl-diacetylene-based difluoromethyl-allo-threonyl-hydroxamate LpxC inhibitors. J Antimicrob Chemother. https://doi.org/10.1093/jac/dkw210
Acknowledgements
JM was supported by the UCSD Graduate Training Program in Cellular and Molecular Pharmacology through an institutional training grant from the National Institute of General Medical Sciences, T32GM007752, and the UCSD Graduate Training Program in Respiratory Biology, T32HL098062. The authors wish to thank the UCSD Histopathology Core facility for their assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Munguia, J., LaRock, D.L., Tsunemoto, H. et al. The Mla pathway is critical for Pseudomonas aeruginosa resistance to outer membrane permeabilization and host innate immune clearance. J Mol Med 95, 1127–1136 (2017). https://doi.org/10.1007/s00109-017-1579-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-017-1579-4