Abstract
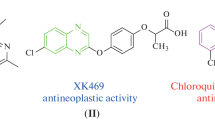
A series of isoxazole linked to 4-(2-oxopyrrolinidyl-1)-tetrahydroquinoline derivatives was efficiently synthesized. The synthetic route started with the formation of the corresponding N-propargyl tetrahydroquinoline derivatives via cationic Povarov reaction. Tetrahydroquinoline-isoxazole hybrid systems (3a–p) were obtained with good yields (42–88%) through a 1,3-dipolar cycloaddition reaction with a click chemistry approach. These compounds have been tested for their in vitro cytotoxic activity against four different human cancer cell lines, lung (A549), liver (HepG2), and melanoma murine (B16F10) using the conventional MTT assay. Among all tetrahydroquinoline-isoxazole hybrids synthesized, compounds 3a, 3e, 3j, and 3m showed promising in vitro activity against HepG2 cancer cell line with considerable selectivity. Compounds 3a (IC50 = 6.80 µM, SI = 14.7) and 3j (IC50 = 5.20 µM, SI > 16.1) exhibited the highest cytotoxic effect. The death pathway related to cytotoxicity of the compound 3j showed necrotic characteristics selectively on the tumor cell line, also showed an improved in vitro activity against the tested reference drug (oxaliplatin), without significant affectation on the viability of hepatocytes. In general, results suggested that these type of hybrid compounds might have therapeutic potential in future investigations on hepatocellular carcinoma.






Similar content being viewed by others
References
Abdel Rahman AM, Ryczko M, Pawling J, Dennis JW (2013) Probing the hexosamine biosynthetic pathway in human tumor cells by multitargeted Tandem Mass Spectrometry. ACS Chem Biol 8:2053–2062
Acelas M, Kouznetsov VV, Romero Bohórquez AR (2018) Facile and highly diastereo and regioselective synthesis of novel octahydroacridine-isoxazole and octahydroacridine-1,2,3-triazole molecular hybrids from citronella essential oil. Mol Divers 23:189–193
Agarwal D, Gupta RD, Awasthi SK (2017) Are antimalarial hybrid molecules a close reality or a distant dream? Antimicrob Agents Chemother 61:1–12
Alfarouk KO, Stock C-M, Taylor S, Walsh M, Muddathir AK, Verduzco D, Bashir AHH, Mohammed OY, Elhassan GO, Harguindey S, Reshkin SJ, Ibrahim ME, Rauch C (2015) Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int 15:71.
Anderson A, Bowman A, Boulton SJ, Manning P, Birch-Machin MA (2014) A role for human mitochondrial complex II in the production of reactive oxygen species in human skin. Redox Biol 2:1016–1022
Alqasoumi SI, Al-Taweel AM, Alafeefy AM, Ghorab MM, Noaman E (2010) Discovering some novel tetrahydroquinoline derivatives bearing the biologically active sulfonamide moiety as a new class of antitumor agents. Eur J Med Chem 45:1849–1853
Al-Sanea MM, El-Deeb IM, Lee SH (2013) Design, synthesis and in-vitro screening of new 1h-pyrazole and 1,2-isoxazole derivatives as potential inhibitors for ROS and mapk14 kinases. Bull Korean Chem Soc 34:437–442
Álvarez Santos MR, Duarte YB, Güiza FM, Romero Bohórquez AR, Mendez-Sanchez SC (2019) Effects of new tetrahydroquinoline-isoxazole hybrids on bioenergetics of hepatocarcinoma Hep-G2 cells and rat liver mitochondria. Chem Biol Interact. https://doi.org/10.1016/j.cbi.2019.02.002
Asolkar RN, Schroeder D, Heckmann R, Lang S, WagnerDoebler I, Laatsch H (2004) Marine bacteria XXVII. Helquinoline, a new tetrahydroquinoline antibiotic from Janibacter limosus Hel 1. J Antibiot 57:17–23
Berghe TV, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P (2014) Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol 15:135–147
Bueno Y, Ramírez JV, Güiza FM, Méndez SC, Romero-Bohórquez AR (2018) Synthesis and in vitro evaluation of cytotoxic effect on cervical cancer cells (HELA) of tetrahydroquinoline-isoxazole hybrid derivatives. Res J Pharm Biol Chem Sci 9:420–432
Champa D, Orlacchio A, Patel B, Ranieri M, Shemetov AA, Verkhusha VV, Cuervo AM, Di Cristofano A (2016) Obatoclax kills anaplastic thyroid cancer cells by inducing lysosome neutralization and necrosis. Oncotarget 7:34453–34471
Chauhan R, Siddiqi AA, Dwivedi J (2012) An approach to regioselective synthesis of pyrazole and isoxazole derivatives and study of their antimicrobial effect. Pharm Chem J 46:316–320
Chen W, Lin Z, Ning M, Yang C, Yan X, Xie Y et al. (2007) Aza analogues of equol: novel ligands for estrogen receptor beta. Bioorg Med Chem 15:5828–5836
Cirrincione G, Barraja P, Diana P, Carbone A, Kelter G, Fiebig H-H (2010) Synthesis and antitumor activity of 2,5-bis(3’-indolyl)-furans and 3,5-bis(3’-indoliy)-isoxazoles, nortopsentin analogues. Bioorg Med Chem 18:4524–4529
Cooper G, Husman R (2007) La célula. Marbán Libros, Madrid
Cury-Boaventura MF, Pompéia C, Curi R (2004) Comparative toxicity of oleic acid and linoleic acid on Jurkat cells. Clin Nutr 23:721–732
Darwish ES, Atia KA, Farag AM (2014) Synthesis and antimicrobial evaluation of some isoxazole based heterocycles. Heterocycles 89:1393–1411
Divakaruni AS, Paradyse A, Ferrick DA, Murphy AN, Jastroch M (2014) Analysis and interpretation of microplate-based oxygen consumption and pH data. Methods Enzymol 547:275–307
Duan Y-T, Man R-J, Tang D-J, Yao Y-F, Tao X-X, Yu C, Liang X-Y, Makawana JA, Zou MJ, Wang Z-C, Zhu H-L (2016) Design, synthesis and antitumor activity of novel link-bridge and B-ring modified combretastatin A-4 (CA-4) analogues as potent antitubulin agents. Sci Rep 6:25387
Eddington ND, Cox DS, Roberts RR, Butcher RJ, Edafiogho IO, Stables JP, Cooke N, Goodwin AM, Smith CA, Scott KR (2002) Synthesis and anticonvulsant activity of enaminones: 4. Investigations on isoxazole derivatives. Eur J Med Chem 37:635–648
Faidallah HM, Rostom S (2013) Synthesis, in vitro antitumor evaluation and DNA-binding study of novel tetrahydroquinolines and some derived tricyclic and tetracyclic ring systems. Eur J Med Chem 63:133–143
Fang X-F, Li D, Reddy VK, Gopala L, Gao W-W, Zhou C-H (2017) Novel potentially antifungal hybrids of 5-flucytosine and fluconazole: design, synthesis and bioactive evaluation. Bioorg Med Chem Lett 27:4964–4969
Fato R, Bergamini C, Bortolus M, Maniero AL, Leoni S, Ohnishi T, Lenaz G (2009) Differential effects of mitochondrial Complex I inhibitors on production of reactive oxygen species. Biochim Biophys Acta 1787:384–392
Fonseca-Berzal C, Merchán DR, Romero Bohórquez AR, Escario JA, Kouznetsov VV, Gómez-Barrio A (2013) Selective activity of 2,4-diaryl-1,2,3,4-tetrahydroquinolines on Trypanosoma cruzi epimastigotes and amastigotes expressing β-galactosidase. Bioorg Med Chem Lett 23:4851–4856
Gao W, Xu K, Li P, Tang B (2011) Funtional roles of superoxide and hydrogen peroxide generated by mitochondrial DNA mutation in regulating tumorigenicity of HepG2 cells. Cell Biochem Funct 29:400–407
Germano D, Tinessa V, Barletta E, Cannella L, Daniele B (2013) Targeted therapy for advanced hepatocellular cancer in the elderly: focus on sorafenib. Drugs Aging 30:887–892
Goli N, Mainkar PS, Kotapalli SS, Tejaswini K, Ummanni R, Chandrasekhar S (2017) Expanding the tetrahydroquinoline pharmacophore. Bioorg Med Chem Lett 27:1714–1720
Gorrini C, Harris IS, Mak T (2013) Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 12:931–947
Hensley CT, Wasti AT, DeBerardinis RJ (2016) Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest 123:3678–3684
Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG (2016) Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 13:714–726
Hou X, Luo H, Zhong H, Wu F, Zhou M, Zhang W, Han X, Yan G, Zhang M, Lu L, Ding Z, He G, Li R (2013) Analysis of furo[3,2-c]tetrahydroquinoline and pyrano[3,2-c]tetrahydroquinoline derivatives as antitumor agents and their metabolites by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 27:1222–1230
Ishiguro H, Toi M (2012) How to dose cytotoxic chemotherapeutic drugs. Curr Top Pharmacool 16:67–81
Kamal A, Bharathi EV, Reddy JS, Ramaiah MJ, Dastagiri D, Reddy MK, Viswanath A, Reddy TL, Shaik TB, Pushpavalli SN, Bhadra MP (2011) Synthesis and biological evaluation of 3,5-diaryl isoxazoline/isoxazole linked 2,3-dihydroquinazolinone hybrids as anticancer agents. Eur J Med Chem 46:691–703
Kerru N, Singh P, Koorbanally N, Raj R, Kumar V (2017) Recent advances (2015–2016) in anticancer hybrids. Eur J Med Chem 142:179–212
Kumar S, Bawa S, Gupta H (2009) Biological activities of quinoline derivatives. Min Rev Med Chem 9:1648–1654
Kumar A, Srivastava S, Gupta G, Chaturvedi V, Sinha S, Srivastava R (2011) Natural product inspired diversity oriented synthesis of tetrahydroquinoline scaffolds as antitubercular agent. ACS Comb Sci 13:65–71
Kumar B, Sharma P, Gupta VP, Khullar M, Singh S, Dogra N, Kumar V (2018) Synthesis and biological evaluation of pyrimidine bridged combretastatin derivatives as potential anticancer agents and mechanistic studies. Bioorg Chem 78:130–140
Kumbhare RM, Kosurkar UB, Janaki Ramaiah M, Dadmal TL, Pushpavalli SN, Pal-Bhadra M (2012) Synthesis and biological evaluation of novel triazoles and isoxazoles linked 2-phenyl benzothiazole as potential anticancer agents. Bioorg Med Chem Lett 22:5424–5427
Lam KH, Lee KK, Gambari R, Wong RS, Cheng GY, Tong SW, Chan KW, Lau FY, Lai PB, Wong WY, Chan AS, Kok SH, Tang JC, Chui CH (2013) Preparation of galipea officinalis hancock type tetrahydroquinoline alkaloid analogues as anti-tumour agents. Phytomedicine 20:166–171
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231
Lin CY, Chang TW, Hsieh WH, Hung MC, Lin IH, Lai SC, Tzeng YJ (2016) Simultaneous induction of apoptosis and necroptosis by Tanshinone IIA in human hepatocellular carcinoma HepG2 cells. Cell Death Discov 2:16065.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2012) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 64:4–17
Liu AL, Shu SH, Qin HL, Lee SM, Wang YT, Du GH (2009) In vitro anti-influenza viral activities of constituents from Caesalpinia sappan. Planta Med 75:337–339
Liu J, Yu LF, Eaton JB, Caldarone B, Cavino K, Ruiz C, Terry M, Fedolak A, Wang D, Ghavami A, Lowe DA, Brunner D, Lukas RJ, Kozikowski AP (2011) Discovery of isoxazole analogues of sazetidine-A as selective α4β2-nicotinic acetylcholine receptor partial agonists for the treatment of depression. J Med Chem 54:7280–7288
Manzione L, Grimaldi AM, Romano R, Ferrara D, Dinota A (2008) Hepatocarcinoma: from pathogenic mechanisms to target therapy. Oncol Rev 2:214–222
Mohammad RM, Muqbil I, Lowe L, Yedjou C, Hsu HY, Lin LT, Siegelin MD, Fimognari C, Kumar NB, Dou QP, Yang H (2015) Broad targeting of resistance to apoptosis in cancer. Semin Cancer Biol 35:S78–S103
Marshall KD, Baines CP (2014) Necroptosis: is there a role for mitochondria? Front Physiol. 5:323.
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Muregi FW, Ishih A (2010) Next-generation antimalarial drugs: hybrid molecules as a new strategy in drug design. Drug Dev Res 71:20–32
Nwaukwa SO, Keehn PM (1989) Ring chlorination of benzenoid compounds using calcium hypochlorite [Ca(OCl)2]. Synth Commun 19:799–804
Palanisamy P, Jenniefer SJ, Muthiah PT, Kumaresan S (2013) Synthesis, characterization, antimicrobial, anticancer, and antituberculosis activity of some new pyrazole, isoxazole, pyrimidine and benzodiazepine derivatives containing thiochromeno and benzothiepino moieties. RSC Adv 3:19300–19310
Palusa SKG, Udupi RH, Himabindu V (2011) Synthesis, antimicrobial and anti-inflammatory studies of isoxazole analogues of rosuvastatin. Der Pharma Chem 3:39–50
Pesi B, Giudici F, Moraldi L, Montesi G, Romagnoli S, Pinelli F, Batignani G (2016) Hepatocellular carcinoma on cirrhosis complicated with tumoral thrombi extended to the right atrium: results in three cases treated with major hepatectomy and thrombectomy under hypothermic cardiocirculatory arrest and literature review. World J Surg Oncol 14:83.
Philipp S, Sosna J, Adam D (2016) Cancer and necroptosis: friend or foe? Cell Mol Life Sci 73:2183–2193
Raymond E, Faivre SJ, Woynarowski JM, Chaney SG (1998) Oxaliplatin: mechanism of action and antineoplastic activity. Semin Oncol 25 2(suppl 5):4–12
Reczek CR, Chandel NS (2017) The two faces of reactive oxygen species in cancer. Annu Rev Cancer Biol 1:4.1–4.20
Reyes-Habito CM, Roh EK (2014) Cutaneous reactions to chemotherapeutic drugs and targeted therapy for cancer: part II. Targeted therapy. J Am Acad Dermatol 71:217.e1–217.e11
Rodríguez YA, Gutiérrez M, Ramírez D, Alzate-Morales J, Bernal CC, Güiza FM, Romero Bohórquez AR (2016) Novel N‐allyl/propargyl tetrahydroquinolines: synthesis via three-component cationic imino Diels–Alder reaction, binding prediction, and evaluation as cholinesterase inhibitors. Chem Biol Drug Des 88:498–510
Romero Bohórquez AR, Escobar P, Leal SM, Kouznetsov VV (2012) In vitro activity against Trypanosoma cruzi and Leishmania chagasi parasites of 2,4-diaryl 1,2,3,4-tetrahydroquinoline derivatives. Lett Drug Des Discov 9:802–808
Rudenko DA, Shavrina TV, Shurov SN, Zykova SS (2014) Synthesis and antioxidant activity of tricyclic compounds containing a 5,6,7,8-tetrahydroquinoline moiety. Pharm Chem J 48:100–103
Sangshetti JN, Khan K, Firoz AK, Abhishek AA, Rohidas P, Rajendra H (2015) Antileishmanial drug discovery: comprehensive review of the last 10 years. RSC Adv 5:32376–32415
Sharma S, Gupta MK, Saxena AK, Singh Bedi PM (2017) Thiazolidinone constraint combretastatin analogs as novel antitubulin agents: design, synthesis, biological evaluation and docking studies. Anticancer Agents Med Chem 17:230–240
Shen YC, Ou DL, Hsu C, Lin KL, Chang CY, Lin CY, Liu SH, Cheng AL (2013) Activating oxidative phosphorylation by a pyruvate dehydrogenase kinase inhibitor overcomes sorafenib resistance of hepatocellular carcinoma. Br J Cancer 108:72–81
Singer JM, Barr BM, Coughenour LL, Walters MA (2005) 8-Substituted 3,4-dihydroquinolinones as a novel scaffold for atypical antipsychotic activity. Bioorg Med Chem Lett 15:4560–4563
Sridharan V, Suryavanshi PA, Menéndez JC (2011) Advances in the chemistry of tetrahydroquinolines. Chem Rev 111:7157–7259
Su Z, Yang Z, Xie L, Dewitt JP, Chen Y (2016) Cancer therapy in the necroptosis era. Cell Death Differ 23:748–756
Sysak A, Obmińska-Mrukowicz B (2017) Isoxazole ring as a useful scaffold in a search for new therapeutic agents. Eur J Med Chem 137:292–309
Tartarone A, Lazzari C, Lerose R, Conteduca V, Improta G, Zupa A, Bulotta A, Aieta M, Gregorc V (2013) Mechanisms of resistance to EGFR tyrosine kinase inhibitors gefitinib/erlotinib and to ALK inhibitor crizotinib. Lung Cancer 81:328–36
Veeraswamy B, Kurumurthy C, Kumar GS, Rao PS, Thelakkat K, Kotamraju S, Narsaiah B (2012) Synthesis of novel 5-substituted isoxazole-3-carboxamide derivatives and cytotoxicity studies on lung cancer cell line. Indian J Chem Sect B 51:1369–1375
Vermes I, Haanen C, Steffens-Nakken H, Reutellingsperger C (1995) A novel assay for apoptosis flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 184:39–51
Wu W, Liu P, Li J (2012) Necroptosis: an emerging form of programmed cell death. Crit Rev Oncol Hematol 82:249–258
Zhang HZ, Zhao ZL, Zhou CH (2018) Recent advance in oxazole-based medicinal chemistry. Eur J Med Chem 144:444–492
Zhao J-J, Yan T, Zhao H, Zhou J-G, Huang Z, Zhang Y-F, Cai J-Q (2015) Evaluation of eight different clinical staging systems associated with overall survival of Chinese patients with hepatocellular carcinoma. Chin Med J 128:316–321
Zhou C, Chen Z, Lu X, Wu H, Yang Q, Xu D (2016) Icaritin activates JNK-dependent mPTP necrosis pathway in colorectal cancer cells. Tumor Biology 37:3135–3144
Zhu Y, Cheng Y, Li A (2013) Mechanisms of drug resistance to sorafenib in hepatocellular carcinoma. Chin Pharmacol Bull 6:752–755
Acknowledgements
This research was supported by “Patrimonio Autónomo, Fondo Nacional de Financiamiento para la Ciencia Francisco José de Caldas” (Colciencias) contract code 110265842934. FMG thanks COLCIENCIAS for his fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher′s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Güiza, F.M., Duarte, Y.B., Mendez-Sanchez, S.C. et al. Synthesis and in vitro evaluation of substituted tetrahydroquinoline-isoxazole hybrids as anticancer agents. Med Chem Res 28, 1182–1196 (2019). https://doi.org/10.1007/s00044-019-02363-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02363-z