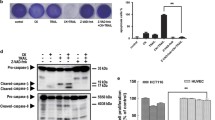
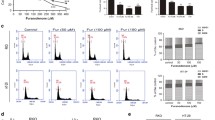
Abstract
The colorectal cancer (CRC) is one leading contributor of cancer-related mortality worldwide. The search for effective anti-CRC agents is valuable. In the current study, we showed that icaritin (ICT), an active natural ingredient from the Chinese plant Epimedium, potently inhibited proliferation and survival of established (HT-29, HCT-116, DLD-1, and SW-620) and primary (patient-derived) CRC cells. Significantly, ICT mainly induced necrosis, but not apoptosis, in CRC cells. The necrosis inhibitor necrostatin-1 attenuated ICT-mediated cytotoxicity in CRC cells. We showed that ICT treatment in CRC cells induced mitochondrial permeability transition pore (mPTP) opening, which was evidenced by mitochondrial membrane potential (MMP) decrease and mitochondrial adenine nucleotide translocator-1 (ANT-1)-cyclophilin-D (CyPD) association. On the other hand, mPTP blockers, including sanglifehrin A, cyclosporin A, and bongkrekic acid, as well as siRNA-mediated knockdown of mPTP component (CyPD or ANT-1), significantly alleviated ICT-mediated cytotoxicity against CRC cells. We suggested that Jun-N-terminal kinase (JNK) activation by ICT mediated mPTP opening and subsequent CRC cell necrosis. JNK pharmacological inhibition, dominant negative mutation, or shRNA downregulation suppressed ICT-induced MMP reduction and subsequent HT-29 cell necrosis. In vivo, oral gavage of ICT dramatically inhibited HT-29 xenograft growth in nude mice. The in vivo activity by ICT was largely attenuated by co-administration with the mPTP blocker CsA. Collectively, our results showed that ICT exerts potent inhibitory effect against CRC cells in vitro and in vivo. JNK-dependent mPTP necrosis pathway could be key mechanism responsible for ICT’s actions.





Similar content being viewed by others
References
Schmoll HJ, Stein A. Colorectal cancer in 2013: towards improved drugs, combinations and patient selection. Nat Rev Clin Oncol. 2014;11:79–80.
Kuipers EJ, Rosch T, Bretthauer M. Colorectal cancer screening—optimizing current strategies and new directions. Nat Rev Clin Oncol. 2013;10:130–42.
Brouquet A, Nordlinger B. Metastatic colorectal cancer outcome and fatty liver disease. Nat Rev Gastroenterol Hepatol. 2013;10:266–7.
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29.
Li C, Li Q, Mei Q, Lu T. Pharmacological effects and pharmacokinetic properties of icariin, the major bioactive component in Herba Epimedii. Life Sci. 2015;126:57–68.
Zhu DY, Lou YJ. Inducible effects of icariin, icaritin, and desmethylicaritin on directional differentiation of embryonic stem cells into cardiomyocytes in vitro. Acta Pharmacol Sin. 2005;26:477–85.
Yao D, Xie XH, Wang XL, Wan C, Lee YW, Chen SH, et al. Icaritin, an exogenous phytomolecule, enhances osteogenesis but not angiogenesis—an in vitro efficacy study. PLoS One. 2012;7:e41264.
Wang Z, Zhang X, Wang H, Qi L, Lou Y. Neuroprotective effects of icaritin against beta amyloid-induced neurotoxicity in primary cultured rat neuronal cells via estrogen-dependent pathway. Neuroscience. 2007;145:911–22.
Huang X, Zhu D, Lou Y. A novel anticancer agent, icaritin, induced cell growth inhibition, G1 arrest and mitochondrial transmembrane potential drop in human prostate carcinoma PC-3 cells. Eur J Pharmacol. 2007;564:26–36.
Guo Y, Zhang X, Meng J, Wang ZY. An anticancer agent icaritin induces sustained activation of the extracellular signal-regulated kinase (ERK) pathway and inhibits growth of breast cancer cells. Eur J Pharmacol. 2011;658:114–22.
Sun L, Chen W, Qu L, Wu J, Si J. Icaritin reverses multidrug resistance of HepG2/ADR human hepatoma cells via downregulation of MDR1 and P-glycoprotein expression. Mol Med Rep. 2013;8:1883–7.
He J, Wang Y, Duan F, Jiang H, Chen MF, Tang SY. Icaritin induces apoptosis of HepG2 cells via the JNK1 signaling pathway independent of the estrogen receptor. Planta Med. 2010;76:1834–9.
Javadov S, Kuznetsov A. Mitochondrial permeability transition and cell death: the role of cyclophilin d. Front Physiol. 2013;4:76.
Halestrap AP, McStay GP, Clarke SJ. The permeability transition pore complex: another view. Biochimie. 2002;84:153–66.
Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis. 2007;12:835–40.
Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM. P53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell. 2012;149:1536–48.
Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans. 2006;34:232–7.
Hausenloy DJ, Lim SY, Ong SG, Davidson SM, Yellon DM. Mitochondrial cyclophilin-D as a critical mediator of ischaemic preconditioning. Cardiovasc Res. 2010;88:67–74.
Chen B, Xu M, Zhang H, Wang JX, Zheng P, Gong L, et al. Cisplatin-induced non-apoptotic death of pancreatic cancer cells requires mitochondrial cyclophilin-D-p53 signaling. Biochem Biophys Res Commun. 2013;437:526–31.
Chen SH, Li DL, Yang F, Wu Z, Zhao YY, Jiang Y. Gemcitabine-induced pancreatic cancer cell death is associated with MST1/cyclophilin D mitochondrial complexation. Biochimie. 2014;103:71–9.
Jerome-Morais A, Bera S, Rachidi W, Gann PH, Diamond AM. The effects of selenium and the GPx-1 selenoprotein on the phosphorylation of H2AX. Biochim Biophys Acta. 2013;1830:3399–406.
Li C, Cui JF, Chen MB, Liu CY, Liu F, Zhang QD, et al. The preclinical evaluation of the dual mTORC1/2 inhibitor INK-128 as a potential anti-colorectal cancer agent. Cancer Biol Ther. 2015;16:34–42.
Qin LS, Yu ZQ, Zhang SM, Sun G, Zhu J, Xu J, et al. The short chain cell-permeable ceramide (C6) restores cell apoptosis and perifosine sensitivity in cultured glioblastoma cells. Mol Biol Rep. 2013;40:5645–55.
Tong JS, Zhang QH, Huang X, Fu XQ, Qi ST, Wang YP, et al. Icaritin causes sustained ERK1/2 activation and induces apoptosis in human endometrial cancer cells. PLoS One. 2011;6:e16781.
Li KR, Zhang ZQ, Yao J, Zhao YX, Duan J, Cao C, et al. Ginsenoside Rg-1 protects retinal pigment epithelium (RPE) cells from cobalt chloride (CoCl2) and hypoxia assaults. PLoS One. 2013;8:e84171.
Qiu Y, Yu T, Wang W, Pan K, Shi D, Sun H. Curcumin-induced melanoma cell death is associated with mitochondrial permeability transition pore (mPTP) opening. Biochem Biophys Res Commun. 2014;448:15–21.
Zecevic A, Menard H, Gurel V, Hagan E, DeCaro R, Zhitkovich A. WRN helicase promotes repair of DNA double-strand breaks caused by aberrant mismatch repair of chromium-DNA adducts. Cell Cycle. 2009;8:2769–78.
Lim SY, Davidson SM, Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: the essential role of the mitochondrial permeability transition pore. Cardiovasc Res. 2007;75:530–5.
Yu T, Li J, Sun H. C6 ceramide potentiates curcumin-induced cell death and apoptosis in melanoma cell lines in vitro. Cancer Chemother Pharmacol. 2010;66:999–1003.
Yang L, Zheng LY, Tian Y, Zhang ZQ, Dong WL, Wang XF, et al. C6 ceramide dramatically enhances docetaxel-induced growth inhibition and apoptosis in cultured breast cancer cells: a mechanism study. Exp Cell Res. 2015;332:47–59.
Elrod JW, Molkentin JD. Physiologic functions of cyclophilin D and the mitochondrial permeability transition pore. Circ J. 2013;77:1111–22.
Zhen YF, Wang GD, Zhu LQ, Tan SP, Zhang FY, Zhou XZ, et al. P53 dependent mitochondrial permeability transition pore opening is required for dexamethasone-induced death of osteoblasts. J Cell Physiol. 2014;229:1475–83.
Clarke SJ, McStay GP, Halestrap AP. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J Biol Chem. 2002;277:34793–9.
Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J Biol Chem. 2005;280:18558–61.
Tang TS, Slow E, Lupu V, Stavrovskaya IG, Sugimori M, Llinas R, et al. Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington’s disease. Proc Natl Acad Sci U S A. 2005;102:2602–7.
Bonora M, Pinton P. The mitochondrial permeability transition pore and cancer: molecular mechanisms involved in cell death. Front Oncol. 2014;4:302.
Crompton M, Barksby E, Johnson N, Capano M. Mitochondrial intermembrane junctional complexes and their involvement in cell death. Biochimie. 2002;84:143–52.
Baines CP. The molecular composition of the mitochondrial permeability transition pore. J Mol Cell Cardiol. 2009;46:850–7.
Lu JH, Shi ZF, Xu H. The mitochondrial cyclophilin D/p53 complexation mediates doxorubicin-induced non-apoptotic death of A549 lung cancer cells. Mol Cell Biochem. 2014;389:17–24.
Acknowledgments
This work was supported by the National Natural Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Chunxian Zhou, Zhengrong Chen, and Xingsheng Lu are co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
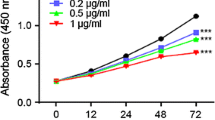
NCM460 cells were either left untreated (“Ctrl”), or treated with applied concentrations of icaritin (ICT) for 72 h, cell proliferation was tested by MTT assay (a), and cell necrosis was tested by LDH release assay (b). Experiments in this figure were repeated three times, and similar results were obtained. For each assay, n = 5. * p < 0.05 vs. “Ctrl” group (GIF 29 kb)
Rights and permissions
About this article
Cite this article
Zhou, C., Chen, Z., Lu, X. et al. Icaritin activates JNK-dependent mPTP necrosis pathway in colorectal cancer cells. Tumor Biol. 37, 3135–3144 (2016). https://doi.org/10.1007/s13277-015-4134-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4134-3