Abstract
Darwin Harbour is a working port and the most populated city in the Northern Territory of Australia. This macrotidal estuary is located in the wet–dry tropics of Northern Australia and notwithstanding mounting development pressures in the region, is largely unmodified. The prevailing oligotrophic condition of estuarine waters suggest that biogeochemical cycling in sediments remain active, buffering the influence of anthropogenic inputs. We tested the hypothesis that nutrient hotspots exist in depositional low-velocity zones, with a gradient of high to low nitrogen processing from the upper to outer reaches of the estuary. A number of factors were examined for their influence on the effectiveness of denitrification in these depositional zones, a putative key process driving nitrogen removal, with particular emphasis on carbon-loading extremes in tidal creeks, spatial gradients along the estuary and the influence of seasonality. There were significant differences in process rates between hypereutrophic/eutrophic tidal creeks that receive the largest proportion of treated sewage loads in the region and the mesotrophic/oligotrophic tidal creeks that were comparatively undisturbed. Net benthic nutrient fluxes and dinitrogen efflux rates were more than an order of magnitude higher and lower, respectively, in degraded (hypereutrophic/eutrophic) tidal creek systems where denitrification efficiency (DE%) was < 40%. Denitrification (Dinitrogen efflux) rates in tidal creeks (mesotrophic/oligotrophic) and broader estuarine sites were high (~ 8 mmol N m−2 day−1) and denitrification efficiency remained > 65%, particularly during the wet season. On a whole-of-estuary basis, denitrification in conjunction with mechanisms such as burial could feasibly make a substantial impact, abating the influence of anthropogenic inputs. Although considerable variability was encountered, particularly across seasons, the hypothesis of elevated denitrification rates as nutrient hotspots in depositional zones along the estuary was not convincing. More influential are tidal creeks as potential ‘reactors’ for N cycling and removal, but their capacity can be degraded by overloading with nutrients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Darwin Harbour is essentially an unmodified macrotidal estuary and one of Australia’s least disturbed working harbours (Munksgaard et al. 2019). The estuary is valued for its intact ecosystems, including extensive mangrove forests, mudflats and reefs, which support an array of wildlife. Worldwide, tropical estuaries are under increasing pressure from anthropogenic impacts with a paucity of research in comparison to temperate counterparts (Gruber and Galloway 2008; Lønborg et al. 2021). This scarcity is even more pronounced for macrotidal systems of the tropics. Darwin Harbour, Australia’s ‘northern gateway’ is also subject to expanding development pressures, and the impact of increasing anthropogenic nutrient loading on ecosystem services is a critical knowledge gap. Impacts are expected to be greater in tropical zones globally (Downing et al. 1999; Galloway et al. 2008; Lara et al. 2001) with regions such as South-East Asia particularly susceptible to anthropogenic nutrient loading, coastal development and climate change pressures (Halpern et al. 2008; Jennerjahn et al. 2004; Lønborg et al. 2021).
Macrotidal estuaries of northern Australia are subject to seasonal extremes with tides of up to 11 m and monsoon conditions driving physical processes with large amounts of suspended material deposited and reworked within the estuary (Andutta et al. 2019). Despite the extreme modulation exerted by tide and climate in Darwin Harbour, the system is largely autotrophic and filterable reactive phosphorus (FRP), combined nitrate and nitrite (NOx) and ammonium (NH4+) are relatively low with means of < 0.05 μmol/l, < 0.13 μmol/l and < 1.1 μmol/l, respectively (DENR 2017, 2018; McKinnon et al. 2006). Molar N:P ratios are < 8 indicating that primary production is likely to be limited by N (Fortune, 2015; McKinnon et al. 2006), and chlorophyll-a concentrations are low (< 2 μg/L) confirming the generally oligotrophic status of the harbour.
Extensive mangrove and mudflat systems fringe the 827 km2 of the Darwin Harbour estuary where sheltered low-velocity zones persist, accumulating fine sediments and organic matter. These intertidal areas provide active sites of remineralisation, releasing inorganic nitrogen and phosphorus to coastal waters (Falcao and Vale 1998; Rocha 1998). Intertidal mangroves act as both sink and source of inorganic N; subsurface sediments have low dissolved oxygen levels and a rich supply of organic matter which favour denitrification. These extensive areas at the interface of the catchment and marine margins are also at greater risk of degradation where population growth and development are likely to be most acute.
Changes to structure and function of tropical estuaries as a consequence of human-induced nutrient loading are well known (Cloern 2001; Howarth 2008; Nixon et al. 1996). So, too, is the importance of benthic and pelagic coupling, where denitrification and nitrification are typically tightly coupled in shallow oligotrophic estuaries like Darwin Harbour (Smith et al. 2012). Eutrophication compromises this balance and the capacity of the system to remove nitrogen. Sediments and benthic communities are at the interface of N processing and most sensitive to nutrient enrichment (Eyre and Ferguson 2009; Viaroli et al. 2004). Benthic denitrification has received much interest as it has the potential to remove a higher proportion of N mineralised within sediments countering the process of eutrophication (Capone et al. 2008; Rysgaard et al. 1995; Seitzinger, 1988). The role of anammox in N removal is considered minimal in estuarine sediments (Dalsgaard et al. 2005; Rich et al. 2008; Risgaard-Petersen et al. 2004) and plays a more pivotal role in deeper marine environments. For most estuaries denitrification appears to propel a large proportion of N2 production. Studies report that anammox can account for < 10% of N removal in estuary sediments (Thamdrup and Dalsgaard 2002; Trimmer et al. 2003; Risgaard-Petersen et al. 2004) compared to higher proportion measured in offshore marine sediments or oxygen minimum zones. Denitrification typically accounts for the bulk N2 loss from intertidal sediments of estuaries, particularly in mangroves (Fernandes et al. 2012; Meyer et al. 2005) where anammox is of minor importance. Also noteworthy is the importance of N-fixation which can serve to offset denitrification in these intertidal sediments where co-occurrence can be responsible, in part for N limitation (Fulweiler et al. 2013). Fringing mangroves of Darwin Harbour are likely to exhibit a large conjunction in the rates of N fixation, denitrification and nitrous oxide flux rates.
Maximum depths up to 36 m are confined to deeper shipping channels of Darwin Harbour with flanking shallower zones and estuarine arms not exceeding 10 m depth. Intertidal sediments examined in this study reside within shallow zones fringed by mudflats and mangroves that are exposed daily by the semi-diurnal macro-tides. For the above-mentioned reasons emphasis for this study is on the importance of benthic denitrification in Darwin Harbour. Benthic biogeochemical processes in Darwin Harbour have been affected by increasing nutrient loads (Smith et al. 2012). Locations of high N loading had compromised denitrification efficiencies; however, this condition was localised. Our understanding of the broader spatial and seasonal importance of denitrification in Darwin Harbour is limited.
The aim of this study was to quantify denitrification of intertidal sediments in Darwin Harbour and evaluate its role in maintaining the harbour’s current oligotrophic condition. The importance of geomorphic zones, such as intertidal mangroves and mudflats, was brought into focus where anthropogenic pressures at these interface areas were most dominant. These intertidal zones are important features in tropical estuaries and highly revered for their cultural, recreational and environmental value. We examined some of the factors which may be influencing the effectiveness of denitrification in these depositional intertidal sediments, with particular emphasis on carbon loading extremes in tidal creeks, spatial gradients along the estuary and the influence of seasonality. To do this, we tested the hypothesis that nutrient hotspots exist in depositional low velocity zones with a gradient of high to low nutrient processing from the upper to outer reaches of estuaries.
Finally, a first-order assessment of areal N2 flux rates for these zones was generated to place this developing tropical harbour in a national and global context.
Materials and methods
Study area
Darwin and vicinity are characterised by high year-round temperatures and a tropical monsoonal climate with a distinct wet and dry season. The mean annual rainfall is approximately 1700 mm, and falls mainly in the wet season between December and March. During the dry season (May–September), the rivers flowing into Darwin Harbour (Blackmore River, Elizabeth River and Howard River) cease to flow, with exception of aquifer-fed residual flow into the Blackmore River and the Howard River. Freshwater runoff in the wet season occurs as discrete flood events during which the upper reaches of the harbour can become entirely fresh. In the upper to middle reaches clear salinity gradients persist (Fortune 2015), in contrast salinity remains almost constant at the mouth of the harbour with freshwater runoff strongly diluted by the time it reaches the mouth (Williams et al. 2006). During the dry season, salinity reflects an almost entirely marine system. The majority of nutrients that enter the harbour are of oceanic origin (Burford et al. 2008) with catchment point and diffuse sources forming a minor contribution. Urban runoff and treated sewage effluent is discharged to the harbour and smaller tidal creeks. Despite these inputs, the harbour is considered to be in essentially natural condition (McKinnon et al. 2006; Munksgaard et al. 2019; Smith et al. 2012).
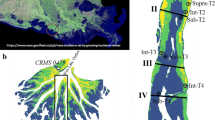
Study sites were located on three tributaries of Shoal Bay to the east of Darwin and three tidal creeks of the Elizabeth River estuary (Fig. 1). The tidal creek systems are typical of many tributaries of Darwin Harbour with margins fringed by dense mangrove forest demarcating bays and estuarine arms. Nine broader estuarine sites on the main arms of Darwin Harbour were also examined to represent more seaward sites along an estuarine to marine continuum (Fig. 1). Seven tidal creek sites were chosen to represent a range of trophic conditions from oligotrophic to hypereutrophic, according to Eyre and Ferguson (2009), to acquire an understanding of the extremes in nutrient loading and benthic fluxes [Fig. 2(a–b)]. Reported critical thresholds for carbon load and denitrification efficiency (DE%) were used to evaluate trophic condition (Eyre and Ferguson 2009). Tidal creek sites were then categorised at two levels, hypereutrophic/eutrophic (those subject to waste discharge) and meso/oligotrophic reflecting the trophic scheme relationship between DE%, carbon loading (fDIC μmol m−2 h−1 as a proxy ƩCO2 flux) and water quality condition. To understand the influence of spatial location and test the hypothesis that a gradient of nutrient processing persists along the estuary, three sites along each estuary arm of Darwin Harbour were chosen. These sites represent upper, middle and outer estuarine intertidal sediments [Fig. 2(c)]. Broader estuarine sites located on the Blackmore, Elizabeth and West arm systems of Darwin Harbour represent typical oligotrophic sites currently not subject to point-source inputs.
Conceptual diagram of hypereutrophic/eutrophic condition a mesotrophic/oligotrophic condition b of tidal creek systems considered in this study and c diagram of spatial location of estuary sites and typical abiotic, nutrient and algal biomass condition. Sites along the estuary represent a gradient of mixing and residence times
Sample collection
Sites were sampled over consecutive years from May 2015 to March 2017 for one wet and dry season each to allow seasonal comparison. Tidal Creek sites (n = 7) were sampled in year 1 (2015–2016) and broader estuarine (n = 9) sites sampled in year 2 (Year 2016–2017). Sediment samples and intact cores were collected at intertidal zones during neap conditions to permit access to sites. Intact sediment cores (n = 96) were collected in triplicate using a pole corer for benthic flux measurements across the field campaign timeframe. Duplicate sediment samples (n = 64) were collected from the top 2 cm and kept on ice until processed.
Water-column physico-chemical conditions
Water-column parameters [temperature, salinity, pH and dissolved oxygen (DO)] were recorded at each site with a Hach Quanta unit (Online Resource Table 2). Photosynthetically active radiation (PAR) was measured during sampling at water-depth intervals of 0.5–1 m using a LI-COR light meter with a spherical quantum underwater sensor (LI-192) and this data used to calculate the euphotic depth. Chlorophyll-a, NOx, NH4+ and Total Nitrogen are drawn from data provided by the Department of Environment and Natural Resources (DENR) monitoring program (DENR 2016, 2017, 2018).
Surface sediments
Surface sediments from each site were transferred into clean glass jars and homogenized. Clean aluminium foil was placed between the lid and contents to minimize organic contamination. The samples were frozen until analysis for Total Organic Carbon (TOC), Total Nitrogen (TN) and subsampled for chlorophyll-a analysis. Although bulk samples were taken from cores to up to 10 cm, the top 2 cm of these sediments was extracted for analysis post-field collection. This data was used for contextual purposes and not included in the analysis.
Benthic flux incubations
Three replicate cores were taken from each site. Cores (7 cm id, 35 cm long) were pressed into the sediment, retaining approximately 1000 ml of overlying water, capped and slowly withdrawn, a plastic plug was then placed in the bottom of the core, before they were returned to the surface. The cores were then returned to the laboratory within 2.5 h, where they were then placed in a water bath and kept at in situ temperature for a pre-incubation period of least 2 h. Core were capped with Plexiglass caps equipped with stirrer and luer-lock feeder lines to a gravity-fed, site-water reservoir to maintain exchange and steady state prior to commencing incubation. Core incubations were carried out according to methods described by Cook et al. 2004a, b; Eyre and Ferguson 2005. Nutrient samples were withdrawn with a plastic syringe and transferred to 10-ml, acid-rinsed and sample-rinsed vials. As a sample was withdrawn, an equal volume was replaced from the gravity-fed reservoir. N2 samples were collected in triplicate by allowing water to flow via individual core feed lines into 7-ml gas-tight glass vials with glass stoppers filled to overflowing. Fluxes were calculated by monitoring the concentration change of individual analytes of N2, NOX, NH4+, DIC and O2 at the start and end of the incubation period for triplicate cores. Samples were taken from the overlying water in the sealed core over the incubation period, which allowed the dissolved oxygen to drop by about 20% from its original in situ value. Concentrations were corrected for the addition of replacement water and any concentration change in a ‘‘blank’’ core containing only water. The flux was calculated as a function of incubation time, core water volume and surface area\(.\)
Negative fluxes denote sediment uptake (influx) and positive fluxes indicate sediment efflux. A net zero flux represents either a balance between analyte production and consumption process in the sediments and/or rates that are below the detection limit. Only dark fluxes were used for analysis as minimal light penetrated to the benthos. In-situ Photosynthetic Active Radiation (PAR) was as low as 0.1 µmol photons m−2 s−1 at the sediment surface during the wet season in tidal creeks and 9 µmol photons m−2 s−1 in more open sites. Mean dry season PAR was 100 µmol photons m−2 s−1. Denitrification efficiency was calculated (excluding cores indicating N2 influxes) as the percentage of inorganic nitrogen (DIN) released from the sediment as dinitrogen gas (N2) during the decomposition of organic matter and calculated as follows:
Analytical methods
Dissolved inorganic nutrients, NH4+ and NOx were drawn from benthic chamber samples filtered and analysed by automated flow injection analysis using standard methods (RSD < 3%). Ammonium was analysed by the automated phenate method and NOx (nitrate and nitrite) by automated cadmium reduction method. Dissolved inorganic nitrogen (DIN) was calculated from the sum of NH4+ and NOx for each sample.
Dissolved inorganic carbon was analysed using a Shimadzu carbon analyzer (Shimadzu TOC-5000A) at the Australian Institute of Marine Science (AIMS) laboratories Townsville (replicate and lab subsamples RSD < 5%). N2 and O2 samples from incubations were measured using a membrane inlet mass spectrometer (MIMS) at the University of Canberra using methods described by Kana et al. 1994. N2 concentrations were determined from changes in the N2:Ar ratios (± 0.05%) with net N2 fluxes reflecting the difference between gross denitrification and gross N fixation (subsequent usage of denitrification in this paper, with N2 efflux from sediments, does not rule out concurrent but lesser N fixation). Ar solubility was corrected for temperature and salinity prior to flux calculations. Sediment oxygen demand (SOD) as dissolved oxygen flux was measured in the cores using a calibrated dissolved oxygen probe (Hach LDO unit ± 5%).
Statistical analysis
Data were analysed using Primer-E 7 (Plymouth, UK), R studio (R v3.2.2) and Sigmaplot (v14). To explore differences in the composite of benthic fluxes between sample groups and their association with abiotic variables, a Euclidean distance matrix was generated based on the normalized benthic flux data (DIC, DO, NH4+, NOx, N2) and visualized with unconstrained principal coordinates analyses (PCO).
Spatial (between creeks), temporal (seasons) or condition-driven (hypereutrophic/eutrophic vs meso/oligotrophic) differences in the fluxes were explored for Year 1 (tidal creeks) and Year 2 (broader estuarine sites) separately. A PERMANOVA (Primer-E v.7) crossed design with type III partial sums of squares and > 990 permutations was used for hypothesis testing with fixed factors season (dry vs wet season) and condition for year 1 with sites nested in condition. Sites were not a random representation of Darwin Harbour but selected based on their distance to the mouth of the creeks, and therefore, also fixed (Quinn and Keough 2002). For year 2 data, season, spatial location (upper, middle and outer) and Estuary were fixed factors. Marginal tests of distance-based linear model (DistLM) in Primer-E were used to determine which normalized abiotic factors best explained the variability in the nutrient fluxes. Abiotic factors were log transformed, normalized and the Euclidean distance matrix generated and visualized with PCOs. Mean values for water quality were also tabulated and mapped in ArcGIS.
Univariate analyses were used to estimate the association of dinitrogen flux with spatial and temporal parameters. Due to the highly heterogenous variance of flux data between seasons, Generalised Least Squares (GLS) models were used with a different variance estimated for the dry and wet season. To account for repeated visits in the dry and wet season to the same sites, a compound symmetry correlation was fitted for sites. The Akaike information criterion (AIC) and Bayesian information criterion (BIC) were used to select models with stepwise variable selection. Standardized model residuals were checked for approximately normal distribution and lack of pattern across explanatory variables.
Results
Physico-chemical conditions
Water column physico-chemical conditions and sediment characteristics for respective sampling years are described in the supplement (Online Resource Tables 3–4, Figs. 7–8). Annual wet-season rainfall for 2015–16 was 1118 mm and 2475 mm for the subsequent sampling year of 2016–17 was recorded at the Darwin Airport gauge (BoM 2019). The Darwin region long-term average is 1684 mm with sampling years representing below and above average years for rainfall. Dry-season water temperatures were generally < 27 °C with higher values during the wet season consistently over 30 °C, particularly for smaller tidal creeks. Water and sediment quality was consistent with the trophic condition spectrum of study sites from hyper/eutrophic extremes to the overwhelmingly representative meso/oligotrophic state.
Benthic metabolism
Mean DIC fluxes ranged from 68 to 406 mmol m−2 day−1, predominantly effluxes and significantly higher at hyper/eutrophic tidal creeks for wet season (Fig. 3). Mean SOD (as O2 flux) varied from − 240 to − 15.1 mmol m−2 day−1, varying across sites and seasons. The most notable SOD fluxes occurred at hypereutrophic to mesotrophic tidal creeks in comparison to broader estuarine sites. These sites also had some of the highest benthic algal biomass (Online Resource, Table 4) which may contribute to this drawdown. Benthic respiration as indicated by oxygen consumption was predominant, particularly at hypereutrophic sites. Benthic metabolism in hyper/eutrophic tidal creeks differed markedly from other sites.
Mean dark wet and dry season benthic fluxes (mmol m−2 day−1) and Denitrification efficiency (%) n = 96. Error bars represent standard error of replicate cores for sites aggregated by condition and estuarine category (Hyereutrophic/eutrophic cores n = 18, Mesotrophic/oligotrophic cores n = 42, Upper estuary cores n = 18, Mid estuary cores n = 18, Outer estuary cores n = 18). A number of samples for NOx and NH4+ were below detection
Influence of trophic condition, estuary zonation and seasonality on N processing
The association between benthic fluxes with season, condition and spatial location for the tidal creeks and estuarine arms was analysed to determine whether the fluxes differed according to trophic condition of tidal creeks. Spatial categories were upper, mid and outer; estuarine zones, and seasonal distinction was made across estuary arms and tidal creeks. Analyses were performed on the composite of benthic fluxes (multivariate analyses) in addition to N2 fluxes alone (univariate analyses).
Trophic condition and N processing in tidal creeks
Benthic nutrient fluxes were notably highest in the hypereutrophic sites of Buffalo Creek (Fig. 3). Dry-season NH4+ fluxes for hypereutrophic sites showed notable benthic influx with values largely negative with a mean of − 21 mmol m−2 day−1 accounting for over 95% of the DIN flux in these systems. Wet season mean NH4+ flux was 28.6 mmol m2 day−1 (Fig. 3) with lesser effluxes observed at the eutrophic site of Myrmidon Creek (Online Resource, Table 6). Notable extremes were observed at the hypereutrophic sites. Ammonium fluxes for meso/oligotrophic tidal creeks ranged from − 2 to 6 mmol m−2 day−1. Mean NH4+ fluxes at meso/oligotrophic sites for dry and wet season were 1.6 mmol m−2 day−1 and 2.3 mmol m−2 day−1, respectively. Mean dry-season NOx fluxes for hyper/eutrophic and meso/oligotrophic sites were 0.5 mmol m−2 day−1, and 0.09 mmol m−2 day−1, respectively. Peak NOx fluxes up to 4.4 mmol m−2 day−1 were measured at hyper/eutrophic sites and up to 2.5 mmol m−2 day−1 at oligotrophic creeks. Wet-season means were lower with influxes predominantly recorded for meso/oligotrophic systems. The low NOx concentrations and values below the limit of detection constrained NOx flux calculations, particularly for estuarine sites.
Wet-season N2 fluxes were variable and highest across tidal creek sites ranging from − 8 to 42.8 mmol m−2 day−1. Influxes were recorded during the dry season at hypereutrophic sites with the exception of one site where N2 efflux exceeded DIN fluxes. Mean dry-season N2 fluxes for meso/oligotrophic and hyper/eutrophic tidal creeks were 0.8 and − 0.5 mmol m−2 day−1, respectively. Wet-season mean N2 fluxes for hyper/eutrophic and meso/oligotrophic tidal creeks were 11 and 8.7 mmol m2 day−1, respectively. Effluxes as high as 53 mmol m−2 day−1 were recorded. Variability was high between triplicate cores with influxes and effluxes measured from within sites. As a consequence a number of negative values constrained denitrification efficiency calculations and were omitted. The mean denitrification efficiency (DE%) for hypereutrophic to eutrophic sites ranged from 3.8 to 62% with the upper value at recorded for the eutrophic site (Myrmidon Creek). Mean DE% across all degraded tidal creeks was typically less than 60% and as low as 30% during dry season.
A PCO ordination of the composite of benthic fluxes showed some clustering of meso/oligotrophic sites, compared to hyper/eutrophic sites (Fig. 4a). The first two PCO axes explained 69.5% of the total variation of the benthic flux data. Impacted tidal creeks were most distinct from meso/oligotrophic sites which is consistent with the significant differences observed in benthic fluxes and pelagic water quality between sites representing the divergent trophic conditions. The more extreme hypereutrophic sites had notably higher DIC, NH4+ and N2 fluxes; these sites also had large SOD.
Sites had a larger effect on the composite of fluxes than trophic condition (multivariate model, Table 1). Differences in flux variability between the trophic conditions also contributed to the observed effects (Table 1, Permdisp results). There was no significant effect of trophic condition upon N2 fluxes in the univariate analysis (Fig. 5, also refer to Online Resource Table 5).
N processing in depositional zones of estuaries
Estuarine NH4+ fluxes were highly variable (− 6 to 21 mmol m−2 day−1) across the continuum of depositional zones. Dry season mean rates for NH4+ in the upper, mid and outer estuarine zones were 1.2, 1.21 and 0 mmol m−2 day−1, respectively. Wet-season means for upper, mid and outer zones were similarly low with influxes for the upper intertidal depositional zones. Mean wet-season NH4+ fluxes for the upper, mid and outer zones were − 1.1, 2.0, and 0.28 mmol m−2 day−1, respectively. NOx fluxes were highly variable and low for most sites, particularly in the upper zones of the estuary where influxes were observed (indicating NOx uptake) unlike mid estuary sites during the dry season (Fig. 3) where a minor efflux of 0.13 mmol m−2 day−1 was observed. The highest NOx flux recorded was 1.2 mmol m−2 day−1 for the Elizabeth River. Across the three estuarine arms of the harbour, mean NOx fluxes ranged from − 0.2 to 0.1 mmol m−2 day−1.
Mean wet-season N2 rates of − 1.5 to 2.9 mmol m−2 day−1 were measured for estuarine sites in the mid to outer zones with the exception of the upper estuarine sites where influx to sediments was observed. Dry-season N2 fluxes for upper, mid and outer sites were low and ranged from 0.008 to 0.01 mmol m−2 day−1 with an overall dry season mean of 0.2 mmol m−2 day−1. Denitrification removed a sizeable portion of nitrogen for the majority of sites, particularly for the estuarine zones with DE% typically > 60%.
Comparison of benthic fluxes with spatial location for broader estuarine sites showed less variation with the exception of a few outliers for the middle and upper location categories. Close clustering of samples in the PCO indicated similarities between all sites along the longitudinal gradient from upper to outer estuary with the exception of one outlier with the first two PCO axes explaining 59% of the flux variation (Fig. 4b). NOx fluxes were either very low or below detection for many sites along the harbour arms. NH4+ was less influential and typically highly variable across the estuary sites (Fig. 3). For the broader estuarine sites, the composite fluxes differed between estuaries and these differences also changed with season (Table 1). There was no evidence that spatial zonation had an impact on the composite fluxes nor N2 fluxes alone (Online Resource, Table 5).
Seasonality in tidal creeks
Composite fluxes clustered according to seasons along the first PCO axis which explained 46.9% of the total variation with the wet season samples more variable and spread out (Fig. 4c). The impact of sites on the composite fluxes significantly varied between the seasons (PERMANOVA P = 0.001, Table 1) and the fluxes differed in their variability between the seasons (PermDisp, P = 0.01, Table 1). Further univariate analysis of N2 fluxes highlighted the differences between the seasons with elevated rates under wet-season conditions (Fig. 5, Online Resource Table 5). N2 fluxes around 0.5 mmol m−2 day−1 were predicted for the dry season.
Seasonality in estuarine arms
Broader estuarine sites indicated diminished influence of seasonality (Fig. 4d) with sites clustered together within the ordination space with the exception of one wet- season outlier. There was little evidence of seasonal influence on fluxes for broader estuarine sites with no difference in N2 fluxes between seasons (Online Resource Table 5). However, the impact of estuary on the composite fluxes differed between the seasons (P = 0.006, Table 1). Results generally reflected tapered benthic fluxes across the estuarine sites and much reduced disparity between wet and dry fluxes and denitrification efficiency. Denitrification efficiency remained high (> 75%) for most estuarine sites under wet season conditions. Estuarine outer zones maintained high denitrification efficiencies across the seasonal extremes.
Discussion
We analysed intertidal sediment fluxes in depositional zones across seasonal, spatial and carbon-loading conditions of a macrotidal estuary in the wet-dry tropics. An emphasis on the N-cycle process of denitrification was explored as an important mechanism that may contribute to the estuary’s assimilatory capacity. We predicted that nutrient hot spots coupled with denitrification would occur along depositional zones in the estuary but instead found that smaller tidal creeks and inlets made a significant contribution to denitrification in Darwin Harbour.
N fluxes across trophic condition spectrum
Nutrient fluxes of degraded tidal creeks, classified as eutrophic or hypereutrophic, were most unlike other sites as a reflection of the differences observed in benthic and pelagic water-quality conditions (Online Resource Table 3, Figs. 7–8). The nutrient fluxes of hypereutrophic/eutrophic tidal creeks showed significant variability in comparison to oligotrophic sites, but tidal creeks broadly aligned with condition, particularly the mesotrophic/oligotrophic category. Enhanced benthic respiration at hypereutrophic/eutrophic sites was likely a consequence of localised enrichment of organic matter and its prolonged retention in these systems, a major factor influencing respiration in shallow coastal systems (Moran and Hodson 1990). Fully functioning benthic (micro-) algae would produce oxygen, but in a turbid hypereutrophic creek, respiration and decomposition is likely to dominate. Furthermore, the ‘benthic microalgae’ are likely to have originated from settled water-column microalgae. Low C:N ratios (Online Resource, Table 4) at degraded sites reflect the predominance of marine algal blooms stimulated by DIN producing autochthonous organic matter, richer in nitrogen.
Notable NH4+ uptake was observed during incubations for the hypereutrophic sites (Online Resource Table 6). However, the stark differences (influxes and effluxes) between hypereutrophic sites and within cores points to the likelihood that the dry season influx is overestimated and/or reflects some contamination. This sediment uptake is inconsistent with a previous evaluation of these impacted sites (Smith et al. 2012). Mangroves are generally DIN sinks (Ray et al. 2014), mostly with NO3− uptake, while NH4+ is frequently released. NH4+ fluxes for meso/oligotrophic systems were mostly undetectable or indicated smaller efflux, suggesting that sediment algal or bacterial N immobilisation is generally effective. Competition for NH4+ between nitrifiers and heterotrophic bacteria is known to have an impact on nitrification rates (Strauss and Lamberti 2000), and consequently denitrification (Risgaard-Petersen 2003). Preferential use of NH4+ by phytoplankton has been previously reported in Darwin Harbour (Burford et al. 2008). Furthermore, the predominance of heterotrophy and anaerobic metabolism producing sulfide may further limit nitrification and denitrification. Any NO3− that is present is available for dissimilatory nitrate reduction to ammonia (DNRA) rather than denitrification under such conditions. Bacteria in the upper reaches of these impacted systems are associated with higher levels of sulfide (Kaestli et al. 2017) signalling further limitation of nitrification–denitrification coupling.
Tidal creek sediments were characterised by high denitrification rates. These interface systems between the catchment and the estuary are often associated with oxygen extremes (suboxic or anoxic sediments), rich carbon environments and delivery of diffuse-source nutrients. These conditions are more likely to support the supply of NO3− and conditions for denitrification. Although the hyper/eutrophic tidal creeks indicated slightly higher denitrification rates (albeit highly variable) in comparison to the meso/oligotrophic systems the GLS analysis for tidal creeks indicated the limited influence of ‘condition’ on N2 fluxes. More convincing was the overwhelming influence of seasonal setting (Fig. 5). The lower denitrification efficiency in hypereutrophic Buffalo Creek is consistent with studies that have demonstrated a smaller proportion of the load removed with enrichment effects (Burford and Longmore 2001; Caffrey et al. 2007; Sloth et al. 1995). Denitrification and nitrification are likely to be decoupled in this system leading to lower denitrification efficiency, a finding supported by previous studies (Smith et al. 2012).
The condition spectrum represented by tidal creeks of this study re-affirms the accepted understanding of nutrient enrichment on denitrification. Observations were consistent with findings of others (Eyre and Ferguson 2009) where critical carbon loads have been defined. The effect of nutrient and organic burden on denitrification efficiency in particular has important implications for the nutrient status of estuarine systems (Kemp et al. 2005; Smith et al. 2012) and their ecological functioning. Further to nutrient status, consistent links with physical properties, residence times, contact with benthos and proximity to catchments in smaller tidal creek systems has been found to have a significant influence (Howarth et al. 1996; Seitzinger et al. 2002) on condition. These factors are likely to play an important role in smaller hydrologically constrained hyper/eutrophic creeks where flushing times are protracted.
N fluxes across estuarine depositional zones
High spatial and temporal variation in measured benthic flux rates has prompted focus on hotspots at the aerobic–anaerobic interface of sediments and associated decomposition of labile organic matter (Fulweiler et al. 2007; Seitzinger and Giblin 1996; Tiedje et al. 1982). This concept underpins our hypothesis of depositional intertidal zones along an estuarine gradient. Comparison of benthic fluxes with spatial location for broader estuarine arm sites showed less variation. Findings were contrary to the expectation of a gradient of influence on benthic fluxes longitudinally within depositional zones, although the factor of ‘Estuary’ (Table 1) indicated some effect. Distinctions between Middle Arm, West Arm and East Arm may explain differences rather than spatial location of sites along individual estuarine arms and their respective gradient. Riverine flows and N loads to these three estuarine systems are distinctive (Fortune et al. 2020).
Trends along the spatial zones were observed for fluxes of DIC and N2, with the upper reach site signalling N-fixation and higher DIC fluxes; however, DE% (> 50%) was similar across the upper, mid and outer reach sites, particularly for the wet season. The lack of measurable nitrate fluxes indicates either low rates of nitrification or close coupling of nitrification and denitrification, where nitrate is quickly processed via denitrification, assimilated by microphytobenthos (MPB) or consumed by high pelagic algal demand (Burford et al. 2008). The microalgal assimilation is likely to be more favourable during the dry season, when light penetration and quality is better. Nitrogen assimilation in the upper reaches, where MPB is notable on mudflats—understood to be depositional areas—is likely to drastically reduce waterborne NH4+ and NO3– concentrations (Anderson et al. 2003; Underwood and Kromkamp 1999). Observations support the premise that in more oligotrophic systems DIN is rapidly assimilated with tight cycling among biotic N pools. Similar findings have been made in subtropical systems in Australia, where it was also found that estuarine sediments generally acted as a sink for nutrients (Ferguson et al. 2004). Mangrove ecosystems surround Darwin Harbour and enhance reassimilation of nutrients by bacteria, which ultimately sequester the nutrients within the sediments (Alongi et al. 1992).
The process of N-fixation on estuarine mudflats yields an abundant source of available N in Darwin Harbour (Burford et al. 2008). Excess of this newly formed N could be conveyed by tidal propagation into the more quiescent tidal creek systems and denitrified there, completing a short N loop back to the atmosphere. Flood tide transfer toward these inlet systems and the protracted residence times therein are likely to favour localised conditions for denitrification. Although N2 effluxes were commonly observed, instances of N2 influxes (n = 20) in tidal creeks in comparison to open estuarine zones (n = 9) also underscore the underlying influence of N-fixation throughout.
It is likely that the heterogeneity of benthic sediments and spring-neap tidal extremes have considerable bearing on the irregularity of N removal within the broader estuary. These processes are undoubtedly actively occurring in small areas or even micro-zones, and across short timescales, for which this study was unable to resolve and may account in some way for the difficulty in resolving denitrification rates at the broader estuarine scale.
N fluxes and seasonality
Seasonality was a critical factor for tidal creeks. GLS analysis revealed the importance of climate extremes experienced by tidal creeks in the region (Fig. 5). Spatial location and season had lesser influence for sites along each estuarine arm in comparison to tidal creeks. This may be explained partly by the disparity in rainfall between sampling years with the model supporting the premise that processes are amplified during the wet season. These extremes in annual rainfall are likely to have had an effect on water quality and the delivery of nutrients and organic loads to the harbour between the two sampling years.
These findings reflect the likelihood that receiving environments of tidal creeks and upper reaches of the estuary arms are subject to the more immediate influence of terrestrial runoff and point sources. Headwaters, such as tidal creeks, are likely to experience these peaks during the wet season. The GLS analysis for tidal creeks suggests increasing wet season variability for predicted N2 fluxes, and a greater influence than trophic condition. The effect of season is distinct and consistent with our current understanding of eutrophication on denitrification and other known regulators (Fulweiler et al. 2007; Seitzinger and Giblin 1996; Seitzinger et al. 2002).
The wet season prompts episodic storm events and the run-off of nitrogen delivered largely in the form of dissolved organic nitrogen, with nitrate dominating DIN species from the catchment (Skinner et al. 2009). Approximately 1747 tonnes of nitrogen enters the estuary from the catchment annually (Fortune et al. 2020), this input currently represents a meagre contribution compared to the large net oceanic import of over 41,000 tonnes per year (Burford et al. 2008). At the seaward interface of the catchment, this load in conjunction with low dissolved oxygen, high turbidity and elevated water temperature characterises typical wet season conditions. One might assume this would increase the rate of denitrification, but in systems where tight coupling of denitrification and nitrification persists, low dissolved oxygen conditions reduce the amount of available nitrate and, therefore, limit denitrification (Jenkins and Kemp 1984). Over the course of a season or seasons, certain areas may switch from prevalence of denitrification to nitrification (Rysgaard et al. 1995) where other transient factors, such as the seasonal abundance of MPB, bioturbation intensity, diel and lunar cycles and tidal variation may influence denitrification rates (Herbert 1999). In our study, sediments of oligotrophic tidal creeks and estuarine sites largely took up NOx in combination with elevated benthic respiration. This may indicate that nitrogen is being immobilised in benthic biomass or removed via denitrification. MPB benefit from increased organic matter decomposition, making use of mineralised inorganic nitrogen released during heterotrophic metabolism (Eyre and Ferguson 2002; Ferguson et al. 2003); however, the lack of light penetration in turbid waters stimulated by season and macrotides limit their productivity.
Denitrification across the estuary
For the estuarine area of Darwin Harbour (827 km2) and with the conservative rate of ~ 5 mmol N m−2 day−1, denitrification could remove > 5000 tonnes/year of N across intertidal zones including depositional mudflats and mangrove zones (Fig. 6). This is likely to be elevated in tidal creeks and mudflat-mangrove systems (~ 153 km2 collectively), where mean N2 fluxes were higher (8–11 mmol N m−2 day−1) causing further N removal. When removal is standardised for area, the tidal creeks contributed disproportionately to N removal (16–51 t km−2 y−1) in comparison to estuarine arms (3.9–6.3 t km−2 y−1). A simplistic extrapolation of rate measurements to sedimentary denitrification removes upwards of 8000 t N/year−1 over the whole estuary based on mean rates across all benthic habitats examined (Fig. 6); however, the variability of rates across time and space suggest even higher rates of removal are probable.
Although considerable variability was encountered across the 2-years study, the hypothesis of elevated denitrification rates as nutrient hotspots in depositional zones along the estuary was not convincing. The study did not support the hypothesis in its entirety; fluxes in depositional zones along the estuary did not reflect a gradient of high to low processing rates. More compelling was the significance of tidal creeks as potential ‘reactors’ for N cycling and removal. These features at the seaward interface of the catchment have emerged as important hotspots, where hydrological flow paths converge with suitable sedimentary substrates and the mangrove zone.
Tidal extremes are an important factor in determining the microbial community in Darwin Harbour (Kaestli et al. 2017), and therefore, an important source of variation driving benthic processing. Further understanding of macrotidal modulation on important ecosystem processes, such as denitrification, would be particularly beneficial. Equally, temporal variation can be significant even over hourly periods. Measuring denitrification across intertidal sediments only during inundation imposes a degree of artificiality on the rates of denitrification measured. Method limitations did not permit resolution around other pathway processes that play a role in the cycling of nitrogen, with emphasis on N removal as a central gap in our understanding. Despite any limitation, the study provides a relative notion of the importance of denitrification across intertidal zones and inlets of Darwin Harbour.
Implications for tropical estuaries
At the interface of land runoff and sea waters, estuaries face compounding impacts of sea level rise, climate change and development pressures. Estuaries are particularly susceptible to anthropogenic N inputs, above all particularly in the tropics (Galloway et al. 1995) and further knowledge is needed to understand the resilience of estuaries. Maintaining effective ecosystem services, such as nutrient cycling, is underpinned by the preservation of intact benthic intertidal communities and mitigating anthropogenic inputs. The further characterisation of these highly active benthic communities for their functional value should be given priority for conservation.
Intertidal mangroves are one such important system in tropical estuaries, which cover 15 million hectares globally and where one-quarter of these communities are moderately to severely degraded (Reef et al. 2010). The action of denitrification and burial can account for considerable assimilation in the mangrove intertidal zone where they act as important sinks (Rivera-Monroy et al. 1995). Limited burial rates are available for Darwin Harbour and more broadly in Northern Australia; however, preliminary data suggest sediment accretion rates of up to 18.5 mm year−1 (Goddard and Hutley, 2019). This implies that burial could further immobilize N, buffering the effects of increasing anthropogenic inputs. In concert with tidal asymmetry producing high rates of deposition within intertidal zones and mangroves (Andutta et al. 2013) burial serves to augment N removal and may well exceed the contribution of denitrification (Eyre et al. 2016).
Denitrification rates for tidal creeks and arms varied significantly in Darwin Harbour. Assuming a mean rate of 2676 μmol N m−2 d−1, on a whole-of-estuary basis, denitrification could feasibly make a substantial impact on ameliorating anthropogenic inputs. Denitrification rates measured elsewhere (Table 2) indicate similar scales of N removal. Studies of tropical Australian systems reflect a wide range of rates from 30 to 12,408 (µmol N m−2 day−1), where those measured for Darwin Harbour are comparable.
Denitrification plays an important role in regulating nitrogen in Darwin Harbour. These findings are consistent with other tropical systems, where microbial denitrification can remove biologically available nitrogen from the water column generating deficits relative to other nutrients (Beman et al. 2005).
Compromised N cycling is largely constrained to small localised sites in Darwin Harbour. However there is growing concern that these conditions could extend beyond the zone of immediate impact as development and population pressures intensify. Inarguably, the effects of marine N pollution are becoming more wide spread and acute globally as a consequence of industrialisation and the intensification of agricultural practices. Although further industrial expansion is touted for places such as Darwin, like most tropical estuaries in Northern Australia, Darwin Harbour is still considered relatively unmodified (Birch et al. 2020; Munksgaard et al. 2019).
Conclusion
This study has extended our understanding of biogeochemical processes across spatial, trophic and seasonal scales in a tropical macro-tidal harbour. We focussed on N cycling with particular emphasis on denitrification to inform knowledge gaps on the importance of this process and its contribution in maintaining the harbour’s current oligotrophic status. Denitrification and benthic fluxes were most affected by nutrient enrichment in tidal creeks in concert with a clear seasonal influence. Legacy and continued enrichment have significantly altered benthic metabolism in localised zones of tidal creeks and could play a considerable role in diminishing nitrogen removal. The notable extremes of the wet season induce distinct abiotic properties of the water column which enhance benthic nutrient cycling during wet-season conditions.
A large proportion of Darwin Harbour’s tidal creeks, like much of Northern Australia, are undisturbed and interfaced with intact mangroves with highly active intertidal sediments. These areas are likely to be important ‘reactors’ or hotspots making a disproportionate contribution to denitrification. Burial is expected to be another important mechanism where tidal asymmetry produces high rates of deposition within intertidal zones. This process would further serve to amplify nitrogen removal in Darwin Harbour. Tidal Creeks are subject to the immediate influence of riverine, overland flows and extended hydraulic residence times. The supply of nitrate in the carbon-rich substrates of mangroves and localised anoxia at the sediment–water interface promote denitrification. These smaller inlet systems warrant further consideration for conservation given the important ecosystem service they provide and their vulnerability to the deleterious effects of emerging development pressures.
This study underlines the important role that microbial communities play in tropical estuaries. Their part in the oxidation of complex organic compounds and regeneration of nutrients to sustain primary production are fundamental and well demonstrated by the cycling of nitrogen. Given the difficulties that often plague conventional measures of denitrification, current trends toward understanding microbial pathways via genomics have come to the fore. The application of functional gene and metagenomic analysis presents exciting opportunities to understand nutrient cycling in these dynamic environments. Such an approach could further corroborate and extend our current biogeochemical understanding of this tropical system.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. Much of the data is distilled within the manuscript or supplementary material.
References
Alongi DM, Boto KG, Robertson AI (1992) Nitrogen and phosphorus cycles. In: Robertson AI, Alongi DM (eds) Tropical mangrove ecosystems. American Geophysical Union, Washington, pp 225–249
Alongi DM, Trott LA, Pfitzner J (2007) Deposition, mineralization, and storage of carbon and nitrogen in sediments of the far northern and northern Great Barrier Reef shelf. Cont Shelf Res 27:2595–2622
Alongi DM, Trott LA, Pfitzner J (2008) Biogeochemistry of inter-reef sediments on the northern and central Great Barrier Reef. Coral Reefs 27:407–420
Anderson IC, McGlathery KJ, Tyler AC (2003) Microbial mediation of ‘reactive’ nitrogen transformations in a temperate lagoon. Mar Ecol Prog Ser 246:73–84
Andutta FP, Wang XH, Li L (2013) Hydrodynamics and sediment transport in a Macro-tidal Estuary: Darwin Harbour Australia. In: Wolanski E (ed) Estuaries of Australia in 2050 and Beyond Estuaries of the world. Springer
Andutta FP, Patterson RG, Wang XH (2019) Monsoon driven waves superpose the effect from macro-tidal currents on sediment resuspension and distribution. Estuar Coast Shelf Sci 223:85–93
Beman JM, Arrigo KR, Matson PA (2005) Agricultural runoff fuels large phytoplankton blooms in vulnerable areas of the ocean. Nature 434:211–214
Birch GF, Lee JH, Tanner E, Fortune J, Munksgaard N, Whitehead J, Coughanowr C, Agius J, Chrispijn J, Taylor U, Wells F, Bellas J, Besada V, Viñas L, Soares-Gomes A, Cordeiro REC, Machado W, Santelli RE, Vaughan M, Cameron M, Brooks P, Crowe T, Ponti M, Airoldi L, Guerra R, Puente A, Gómez AG, Zhou GJ, Leung KMY, Steinberg P (2020) Sediment metal enrichment and ecological risk assessment of ten ports and estuaries in the World Harbours Project. Mar Pollut Bull 155:111129. https://doi.org/10.1016/j.marpolbul.2020.111129
BoM (2019) Bureau of Meteorology. Monthly rainfall data, Darwin Airport. Product Code: IDCJAC0001 reference: 67229272. http://www.bom.gov.au/tmp/cdio/IDCJAC0001_014015.pdf. Accessed 4 December 2021
Burford MA, Longmore AR (2001) High ammonium production from sediments in hypereutrophic shrimp ponds. Mar Ecol Prog Ser 224:187–195
Burford MA, Alongi DM, McKinnon D, Trott LA (2008) Primary production and nutrients in a tropical macrotidal estuary, Darwin Harbour, Australia. Estuar Coast Shelf Sci 79(3):440–448
Caffrey JM, Kemp WM (1990) Nitrogen cycling in sediments with estuarine populations of Potamogeton perfiolatus and Zostera marina. Mar Ecol Prog Ser 66:147–160
Caffrey JM, Murrell MC, Wigand C, McKinney R (2007) Effect of nutrient loading on biogeochemical and microbial processes in a New England salt marsh. Biogeochemistry 82(3):251–264
Capone DG, Carpenter EJ, Bronk DA, Mulholland MR (2008) Nitrogen in the marine environment. Elsevier Science and Technology
Cloern JE (2001) Our evolving conceptual model of the coastal eutrophication problem. Mar Ecol Prog Ser 210:223–253
Cook PLM, Eyre BD, Leeming R, Butler ECV (2004a) Benthic fluxes of nitrogen in the tidal reaches of a turbid, high-nitrate sub-tropical river. Estuar Coast Shelf Sci 59(4):675–685
Cook PLM, Revill A, Butler ECV, Eyre BD (2004b) Carbon and nitrogen cycling on intertidal mudflats of a temperate Australian estuary: II. Nitrogen cycling. Mar Ecol Prog Ser 280:39–54
Dalsgaard T, Thamdrup B, Canfield DE (2005) Anaerobic ammonium oxidation (anammox) in the marine environment. Res Microbiol 156:457–464
DENR (2016) Darwin Harbour Region Report Card 2016. Aquatic Health Unit, Department of Environment and Natural Resources Northern Territory Government. Darwin Australia
DENR (2017) Darwin Harbour Region Report Card 2017. Aquatic Health Unit, Department of Environment and Natural Resources Northern Territory Government. Darwin Australia
DENR (2018) Darwin Harbour Region Report Card 2018. Aquatic Health Unit, Department of Environment and Natural Resources Northern Territory Government. Darwin Australia
Downing JA, Mcclain M, Twilley R, Melack JM, Elser J, Rabalais NN, Lewis WM, Turner RE, Corredor J, Soto D, Yanez-Arancibia A, Kopaska JA, Howarth RW (1999) The impact of accelerating land-use change on the N-Cycle of tropical aquatic ecosystems: current conditions and projected changes. Biogeochemistry 46(1–3):109–148
Eyre BD, Ferguson AJP (2002) Comparison of carbon production and decomposition, benthic nutrient fluxes and denitrification in seagrass, phytoplankton, benthic microalgae- and macroalgae-dominated warm-temperate Australia lagoons. Mar Ecol Prog Ser 229:43–59
Eyre BD, Ferguson AJP (2005) Benthic metabolism and nitrogen cycling in a subtropical east Australian estuary (Brunswick): temporal variability and controlling factors. Limnol Oceanogr 50(1):81–96
Eyre BD, Ferguson AJP (2009) Denitrification efficiency for defining critical loads of carbon in shallow coastal ecosystems. Hydrobiologia 629:137–146
Eyre BD, Santos IR, Maher DT (2013) Seasonal, daily and diel N2 effluxes in permeable carbonate sediments. Biogeosciences 10(4):2601–2615. https://doi.org/10.5194/bg-10-2601-2013
Eyre BD, Maher DT, Sanders C (2016) The contribution of denitrification and burial to the nitrogen budgets of three geomorphically distinct Australian estuaries: importance of seagrass habitats. Limnol Oceanogr 61:114–1156
Falcão M, Vale C (1998) Sediment–water exchanges of ammonium and phosphate in intertidal and subtidal areas of a mesotidal coastal lagoon (Ria Formosa). Hydrobiologia 373:193–201. https://doi.org/10.1023/A:1017083724636
Ferguson A, Eyre BD, Gay J (2003) Organic matter and benthic metabolism in euphotic sediments along shallow sub-tropical estuaries, northern New South Wales, Australia. Aquat Microb Ecol 33:137–154
Ferguson A, Eyre BD, Gay J (2004) Nutrient cycling in the sub-tropical Brunswick Estuary, Australia. Estuaries 27(1):1–17
Fernandes SO, Michotey VD, Guasco S, Bonin PC, Loka Bharathi PA (2012) Denitrification prevails over anammox in tropical mangrove sediments (Goa, India). Mar Environ Res 74:9–19
Fortune J (2015) Spatial variability of Darwin Harbour water quality during dry season neap tides of 2012 and 2013. Report No. 16/2015D. Aquatic Health Unit, Department of Land Resource Management
Fortune J, Butler ECV, Gibb K (2020) A decade of nitrogen inputs to a tropical macrotidal estuary of Northern Australia, Darwin harbour. Reg Stud Mar Sci 36:101275
Fulweiler RW, Nixon SW, Buckley BA, Granger SL (2007) Reversal of the net dinitrogen gas flux in coastal marine sediments. Nature 448:180–182
Fulweiler RW, Brown SM, Nixon SW, Jenkins BD (2013) Evidence of a conceptual model for the co-occurrence of nitrogen fixation and denitrification in heterotrophic marine sediments. Mar Ecol Prog Ser 482:57–68
Galloway JN, Schlesinger WH, Levy V, Michaels A, Schnoor JL (1995) Nitrogen fixation: anthropogenic enhancement-environmental response. Global Biogeochem Cycles 9(2):235–252
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320(5878):889–892
Gardner WS, McCarthy MJ (2009) Nitrogen dynamics at the sediment-water interface in shallow, sub-tropical Florida Bay: why denitrification efficiency may decrease with increased eutrophication. Biogeochemistry 95:185–198
Goddard MM, Hutley LB (2019) Development of a long-term sediments monitoring program in Darwin Harbour: A final report on the Rod Surface Elevation Table – Marker Horizon Pilot project MP2, Report No. 37/2019. A report for the Northern Territory Government, Department of Environment and Natural Resources, NT
Gruber N, Galloway JN (2008) An Earth-system perspective of the global nitrogen cycle. Nature 451:293–295. https://doi.org/10.1038/nature06592
Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, D’Agrosa C, Bruno JF, Casey KS, Ebert C, Fox HE, Fujita R, Heinemann D, Lenihan HS, Madin EMP, Perry MT, Selig ER, Spalding M, Steneck R, Watson R (2008) A global map of human impact on marine ecosystems. Science 319:948–952
Herbert R (1999) Nitrogen cycling in coastal marine ecosystems. FEMS Microbiol Rev 23(5):563–590
Howarth RW (2008) Coastal nitrogen pollution: a review of sources and trends globally and regionally. Harmful Algae 8:14–20
Howarth RW, Billen G, Swaney D, Townsend A, Jaworski N, Lajtha K, Downing JA, Elmgren R, Caraco N, Jordan T, Berendse F, Freney J, Kudeyarov V, Murdoch P, Zhao-Liiang Z (1996) Regional nitrogen budgets and riverine N and P fluxes for the drainages to the North Atlantic Ocean: natural and human influences. Biogeochemistry 35:75–139
Jenkins MC, Kemp WM (1984) The coupling of nitrification and denitrification in two estuarine sediments. Limnol Oceanogr 29:609–619
Jennerjahn TC, Ittekkot V, Klöpper S, Seno A, Purwo Nugroho S, Sudiana N, Anyuta Y, Prihartanto G-H (2004) Biogeochemistry of a tropical river affected by human activities in its catchment: Brantas River estuary and coastal waters of Madura Strait, Java, Indonesia. Estuar Coast Shelf Sci 60(3):503–514
Joye SB, Paerl HW (1994) Nitrogen cycling in microbial mats: rates and patterns of denitrification and nitrogen fixation. Mar Biol 119:285–295
Kaestli M, Skillington A, Kennedy K, Majid M, Williams D, McGuiness K, Munksgaard N, Gibb K (2017) Spatial and temporal microbial patterns in a tropical macotidal estuary subject to urbanisation. Front Microbiol 8:1313
Kana TM, Darkangelo C, Hunt MD, Oldham JB, Bennett GE, Cornwell JC (1994) Membrane inlet mass spectrometer for rapid high-precision determination of N2, 02, and Ar in environmental water samples. Anal Chem 66:4166–4170
Kemp WM, Boynton WR, Adolf JE, Boesch DF, Boicourt W, Brush G, Cornwell JC, Fisher TR, Glibert PM, Hagy JD, Harding LW, Houde ED, Kimmel DG, Miller WD, Newell RIE, Roman MR, Smith EM, Stevenson JC (2005) Eutrophication of Chesapeake Bay: historical trends and ecological interactions. Mar Ecol Prog Ser 303:1–29
Lara LBLS, Artaxob P, Martinellia LA, Victoria RL, Camargo PB, Krusche A, Ayers GP, Ferraz ESB, Ballester MV (2001) Chemical composition of rainwater and anthropogenic influences in the Piracicaba River Basin, Southeast Brazil. Atmos Environ 35:4937–4945
Lønborg C, Müller M, Butler ECV, Jiang S, Ooi SK, Trinh DH, Wong PY, Ali SM, Chun C, Wee BS, Yando ES (2021) Nutrient cycling in tropical and temperate coastal waters: is latitude making a difference? Estuar Coast Shelf Sci 262:107571
McKinnon AD, Smit N, Townsend S, Duggan S (2006) Darwin Harbour: water quality and ecosystem structure in a tropical harbour in the early stages of development. In: Wolanski E (ed) The environment in Asia pacific harbours. Springer, Dordrecht, pp 433–459
Meyer RL, Risgaard-Petersen N, Allen DE (2005) Correlation between anammox activity and microscale distribution of nitrite in a subtropical mangrove sediment. Appl Environ Microbiol 71:6142–6149
Moran MA, Hodson RE (1990) Bacterial production on humic and non-humic components of dissolved organic carbon. Limnol Oceanogr 35:1744–1756
Munksgaard NC, Hutley LB, Metcalfe KN, Padovan AC, Palmer C, Gibb KS (2019) Environmental challenges in a near-pristine mangrove estuary facing rapid urban and industrial development: Darwin harbour, Northern Australia. Reg Stud Mar Sci 25:100438
Nishio T, Koike I, Hattori A (1983) Estimates of denitrification and nitrification in coastal and estuarine sediments. Appl Environ Microbiol 45:444–450
Nixon SW, Ammerman JW, Atkinson LP, Berounsky VM, Billen G, Boicourt WC, Boynton WR, Church TM, Ditoro DM, Elmgren R, Garber JH, Giblin AE, Jahnke RA, Owens NJP, Pilson MEQ, Seitzinger SP (1996) The fate of nitrogen and phosphorus at the land-sea margin of the North Atlantic Ocean. Biogeochemistry 35(1):141–180
Nowicki BL (1994) The effect of temperature, oxygen, salinity and nutrient enrichment on estuarine denitrification rates measured with a modified nitrogen gas flux technique. Est Coast Shelf Sci 38:137–156
Ogilvie BG, Nedwell DB, Harrison RM, Robinson AD, Sage AS (1997) High nitrate, muddy estuaries as nitrogen sinks: the nitrogen budget of the River Colne estuary. Mar Ecol Prog Ser 150:217–228
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Radke LC, Fortune J, Majid M, Mummery A (2020) Sediment quality assessment of East Arm, Darwin Harbour (2019): Survey record and data report. Technical Report No. 9/2020, Department of Environment and Natural Resources, Northern Territory Government, NT
Ray R, Majumder N, Das S, Chowdhury C, Jana TK (2014) Biogeochemical cycle of nitrogen in a tropical mangrove ecosystem, east coast of India. Mar Chem 167:33–43
Reef R, Feller IC, Lovelock CE (2010) Nutrition of mangroves. Tree Physiol 30:1148–1160
Rich JJ, Dale OR, Song B, Ward BB (2008) Anaerobic ammonium oxidation (Anammox) in Chesapeake Bay sediments. Microb Ecol 55:311–320
Risgaard-Petersen N (2003) Coupled nitrification-denitrification in autotrophic and heterotrophic estuarine sediments: on the influence of benthic microalgae. Limnol Oceanogr 48:93–105
Risgaard-Petersen N, Nicolaisen MH, Revsbech NP, Lomstein BA (2004) Competition between ammonia-oxidizing bacteria and benthic microalgae. Appl Environ Microbiol 70:5528–5537
Rivera-Monroy VH, Twilley RR, Boustany RG, Day JW, Vera-Herra F, del Carmen RM (1995) Direct denitrification in mangrove sediments in Terminos Lagoon, Mexico. Mar Ecol Prog Ser 126:97–109
Rocha C (1998) Rhythmic ammonium regeneration and flushing in intertidal sediments of the Sado Estuary. Limnol Oceanogr 43(5):823–831
Rysgaard S, Christensen PB, Nielsen LP (1995) Seasonal variation in nitrification and denitrification in estuarine sediment colonized by benthic microalgae and bioturbating infauna. Mar Ecol Prog Ser 126:111–121
Seitzinger SP (1988) Denitrification in freshwater and coastal marine ecosystems: ecological and geochemical significance. Limnol Oceanogr 33(4 part 2):702–724
Seitzinger SP, D’Elia CF (1985) Preliminary studies of denitrification on a coral reef, in: The ecology of coral reefs, Symposia series for undersea research, edited by: Reaka, M. L., National Oceanic Atmospheric Administration: Department of Commerce, Washington, DC, 194–208
Seitzinger SP, Giblin AE (1996) Estimating denitrification in North Atlantic continental shelf sediments. Biogeochemistry 35:235–260
Seitzinger SP, Styles RV, Boyer EW, Alexander RB, Billen G, Howrath R, Mayer B, Van Breeman N (2002) Nitrogen retention in rivers: model development and application to watersheds in the northeastern USA. Biogeochemistry 57:199–237
Skinner L, Townsend SA, Fortune J (2009) The impact of urban land-use on total pollutant loads entering Darwin harbour Report 06/2008D. Department of Environment and Natural Resources Northern Territory Government Australia
Sloth NP, Blackburn H, Hansen LS, Risgaard-Petersen N, Lomstein BA (1995) Nitrogen cycling in sediment with different organic loading. Mar Ecol Prog Ser 116:163–170
Smith J, Burford MA, Revill AT, Haese RR, Fortune J (2012) Effect of nutrient loading on biogeochemical processes in tropical tidal creeks. Biogeochemistry 108(1–3):359–380
Strauss EA, Lamberti GA (2000) Regulation of nitrification in aquatic sediments by organic carbon. Limnol Oceanogr 45(8):1854–1859
Thamdrup B, Dalsgaard T (2002) Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Appl Environ Microbiol 68(3):1312–8
Tiedje J, Sexstone AJ, Myrold DD, Robinson JA (1982) Denitrification: ecological niches, competition and survival. Antonie Leeuwenhoek 48:569–583
Trimmer M, Nicholls JC, Deflandre B (2003) Anaerobic ammonium oxidation measured in sediments along the Thames estuary, United Kingdom. Appl Environ Microbiol 69:6447–6454
Underwood GJC, Kromkamp J (1999) Primary production by phytoplankton and microphytobenthos in estuaries. Adv Ecol Res 29:93–139
Viaroli P, Bartoli M, Giordani G, Magni P, Welsh DT (2004) Biogeochemical indicators as tools for assessing sediment quality/vulnerability in transitional aquatic ecosystems. Aquatic Conserv: Mar Freshw Ecosyst 14:S19–S29
Williams D, Wolanski E, Spagnol S (2006) Hydrodynamics of Darwin harbour. In: Wolanski E (ed) The environment in Asia pacific harbours. Springer, Dordrecht, pp 461–476
Acknowledgements
We acknowledge funding support for this project by Santos Australia and the NT Department of Environment, Parks and Water Security. We thank Matthew Majid and James Wyatt of the Aquatic Health Unit, Northern Territory Government, Larrakia Sea Rangers for field support and the Australian Institute of Marine Science, Southern Cross University and Canberra University for their laboratory services and support. We are very grateful for the insights and comments from anonymous reviewers.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Santos Pty Ltd provided a Research Grant to support the project coordinated by Julia Fortune.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fortune, J., Kaestli, M., Butler, E.C.V. et al. Denitrification in intertidal sediments of a tropical estuary subject to increasing development pressures. Aquat Sci 84, 53 (2022). https://doi.org/10.1007/s00027-022-00885-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-022-00885-0