Abstract
Introduction
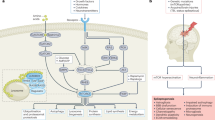
Mitochondrial dysfunction is a common denominator of neuroinflammation recognized by neuronal oxidative stress-mediated apoptosis that is well recognized by common intracellular molecular pathway-interlinked neuroinflammation and mitochondrial oxidative stress, a feature of epileptogenesis. In addition, the neuronal damage in the epileptic brain corroborated the concept of brain injury-mediated neuroinflammation, further providing an interlink between inflammation, mitochondrial dysfunction, and oxidative stress in epilepsy.
Materials and methods
A systematic literature review of Bentham, Scopus, PubMed, Medline, and EMBASE (Elsevier) databases was carried out to provide evidence of preclinical and clinically used drugs targeting such nuclear, cytosolic, and mitochondrial proteins suggesting that the correlation of mechanisms linked to neuroinflammation has been elucidated in the current review. Despite that, the evidence of elevated levels of inflammatory mediators and pro-apoptotic protein levels can provide the correlation of inflammatory responses often concerned with hyperexcitability attributing to the fact that mitochondrial redox mechanisms and higher susceptibilities to neuroinflammation result from repetitive recurring epileptic seizures. Therefore, providing an understanding of seizure-induced pathological changes read by activating neuroinflammatory cascades like NF-kB, RIPK, MAPK, ERK, JNK, and JAK-STAT signaling further related to mitochondrial damage promoting hyperexcitability.
Conclusion
The current review highlights the further opportunity for establishing therapeutic interventions underlying the apparent correlation of neuroinflammation mediated mitochondrial oxidative stress might contribute to common intracellular mechanisms underlying a future prospective of drug treatment targeting mitochondrial dysfunction linked to the neuroinflammation in epilepsy.



Similar content being viewed by others
Availability of data and materials
Not applicable.
Abbreviations
- ERK:
-
Extracellular regulated kinase
- NF-κB:
-
Nuclear factor-kappa light chain enhancer of activated B cells
- JAK–STAT:
-
Janus kinases signal transducer and activator of transcription proteins
- MAPK:
-
Mitogen-activated protein kinases
- P38:
-
P38 MAP KINASE
- PPARs:
-
Peroxisome proliferator-activated receptors
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- AMPK:
-
AMP-activated protein kinase
- SIRT1:
-
Silent mating type information regulation 2 homolog
- PGC-1-alpha:
-
Peroxisome proliferator-activated receptor-γ
- COX-2:
-
Cyclooxygenase2
- ASK-1:
-
Apoptosis signal-regulating kinase-1
- TNF α:
-
Tumor necrosis factor alpha
- IL-1:
-
Interleukin-1
- INOS:
-
Inducible nitric oxide synthase
- PGC-1α:
-
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- UCP2:
-
Uncoupling protein 2
- CCL2:
-
Chemokine (C–C motif) ligand 2
- MnSOD:
-
Manganese-dependent superoxide dismutase
- DNA:
-
Deoxyribonucleic acid
- NAD:
-
Nicotinamide adenine dinucleotide
- FOXO:
-
Forkhead box transcription factors
References
Brown GC, Murphy MP, Jornayvaz FR, Shulman GI. Regulation of mitochondrial biogenesis. Essays Biochem. 2010;47:69–84. https://doi.org/10.1042/bse0470069.
Onyango IG, Lu J, Rodova M, Lezi E, Crafter AB, Swerdlow RH. Regulation of neuron mitochondrial biogenesis and relevance to brain health. Biochim Biophys Acta Mol Basis Dis. 2010;1802:228–34. https://doi.org/10.1042/bse0470069.
Udhayabanu T, Manole A, Rajeshwari M, Varalakshmi P, Houlden H, Ashokkumar B. Riboflavin responsive mitochondrial dysfunction in neurodegenerative diseases. J Clin Med. 2017. https://doi.org/10.3390/jcm6050052.
Zsurka G, Kunz WS. Mitochondrial dysfunction and seizures: the neuronal energy crisis. Lancet Neurol. 2015;14:956–66. https://doi.org/10.1016/S1474-4422(15)00148-9.
Kovac S, Dinkova Kostova AT, Herrmann AM, Melzer N, Meuth SG, Gorji A. Metabolic and homeostatic changes in seizures and acquired epilepsy—mitochondria, calcium dynamics and reactive oxygen species. J Mol Sci. 2017;18:1935. https://doi.org/10.3390/ijms18091935.
Singh S, Singh TG, Rehni AK. An insight into molecular mechanisms and novel therapeutic approaches in epileptogenesis. CNS Neurol Disord Drug Targets. 2020;19(10):750–79. https://doi.org/10.2174/1871527319666200910153827.
Yang H, Wu J, Guo R, Peng Y, Zheng W, Liu D, Song Z. Glycolysis in energy metabolism during seizures. Neural Regen Res. 2013;8:1316. https://doi.org/10.3969/j.issn.1673-5374.2013.14.008.
Kovács R, Gerevich Z, Friedman A, Otáhal J, Prager O, Gabriel S, Berndt N. Bioenergetic mechanisms of seizure control. Front Cell Neurosci. 2018;12:335. https://doi.org/10.3389/fncel.2018.00335.
Sharma VK, Singh TG, Mehta V. Stressed mitochondria: A target to intrude Alzheimer’s disease. Mitochondrion. 2021;59:48–57. https://doi.org/10.1016/j.mito.2021.04.004.
McDonald T, Puchowicz M, Borges K. Impairments in oxidative glucose metabolism in epilepsy and metabolic treatments thereof. Front Cell Neurosci. 2018;12:274. https://doi.org/10.3389/fncel.2018.00274.
Gleichmann M, Mattson MP. Neuronal calcium homeostasis and dysregulation. Antioxid Redox Signal. 2011;14:1261–73. https://doi.org/10.1089/ars.2010.3386.
Roganovic M, Pantovic S, Dizdarevic S. Role of the oxidative stress in the pathogenesis of epilepsy. Brain. 2019;1:3. https://doi.org/10.5152/NSN.2019.11632.
López-Armada MJ, Riveiro-Naveira RR, Vaamonde-García C, Valcárcel-Ares MN. Mitochondrial dysfunction and the inflammatory response. Mitochondrion. 2013;13:106–18. https://doi.org/10.1016/j.mito.2013.01.003.
Rose J, Brian C, Woods J, Pappa A, Panayiotidis MI, Powers R, Franco R. Mitochondrial dysfunction in glial cells: implications for neuronal homeostasis and survival. Toxicology. 2017;391:109–15. https://doi.org/10.1016/j.tox.2017.06.011.
Meng XF, Tan L, Tan MS, Jiang T, Tan CC, Li MM, Wang HF, Yu JT. Inhibition of the NLRP3 inflammasome provides neuroprotection in rats following amygdala kindling-induced status epilepticus. J Neuroinflamm. 2014;11:1–2. https://doi.org/10.1186/s12974-014-0212-5.
Rehni AK, Singh TG, Chand P. Amisulpride-induced seizurogenic effect: a potential role of opioid receptor-linked transduction systems. Basic Clin Pharmacol Toxicol. 2011;108(5):310–7. https://doi.org/10.1111/j.1742-7843.2010.00655.x.
Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun. 2011;25:1281–9. https://doi.org/10.1016/j.bbi.2011.03.018.
Brodie MJ. Antiepileptic drug therapy the story so far. Seizure. 2010;19:650–5. https://doi.org/10.1016/j.seizure.2010.10.027.
Guimarães J, Ribeiro JA. Pharmacology of antiepileptic drugs in clinical practice. Neurologist. 2010;16:353–7. https://doi.org/10.1097/NRL.0b013e3181dba5d3.
Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–5. https://doi.org/10.1038/sj.embor.7400779.
Sugiura Y, Ugawa Y. Epilepsy and ion channels. Clin Neurol. 2016;16(57):1–8. https://doi.org/10.5692/clinicalneurol.cn-000963.
Nigar S, Pottoo FH, Tabassum N, Verma SK, Javed MN. Molecular insights into the role of inflammation and oxidative stress in epilepsy. J Adv Med Pharm Sci. 2016;12:1–9. https://doi.org/10.9734/JAMPS/2016/24441.
Waldbaum S, Patel M. Mitochondrial dysfunction and oxidative stress: a contributing link to acquired epilepsy? J Bioenerg Biomembr. 2010;1(42):449–55. https://doi.org/10.1007/s10863-010-9320-9.
Pu X, Storr SJ, Zhang Y, Rakha EA, Green AR, Ellis IO, Martin SG. Caspase-3 and caspase-8 expression in breast cancer: caspase-3 is associated with survival. Apoptosis. 2017;22:357–68. https://doi.org/10.1007/s10495-016-1296-4.
Maroso M, Balosso S, Ravizza T, Liu J, Bianchi ME, Vezzani A. Interleukin-1 type 1 receptor/Toll-like receptor signalling in epilepsy: the importance of IL-1beta and high-mobility group box 1. J Intern Med. 2011;270:319–26. https://doi.org/10.1111/j.1365-2796.2011.02431.x.
Rana A, Musto AE. The role of inflammation in the development of epilepsy. J Neuroinflamm. 2018;15:1–2. https://doi.org/10.1186/s12974-018-1192-7.
Lalani AI, Zhu S, Gokhale S, Jin J, Xie P. TRAF molecules in inflammation and inflammatory diseases. Curr Pharmacol Rep. 2018. https://doi.org/10.1007/s40495-017-0117-y.
Zhang XM, Zhu J. Kainic acid-induced neurotoxicity: targeting glial responses and glia-derived cytokines. Curr Neuropharmacol. 2011;9:388–98. https://doi.org/10.2174/157015911795596540.
Rehni AK, Singh TG, Kalra R, Singh N. Pharmacological inhibition of inducible nitric oxide synthase attenuates the development of seizures in mice. Nitric Oxide. 2009;21(2):120–5. https://doi.org/10.1016/j.niox.2009.06.001.
Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–35. https://doi.org/10.1016/j.tins.2007.07.007.
Scott RC. What are the effects of prolonged seizures in the brain? Epileptic Disord. 2014;16:S6-11. https://doi.org/10.1684/epd.2014.0689.
Sanz P, Garcia-Gimeno MA. Reactive glia inflammatory signaling pathways and epilepsy. Int J Mol Sci. 2020;21:4096. https://doi.org/10.1016/j.mito.2021.03.009.
Singh S, Singh TG, Rehni AK, Sharma V, Singh M, Kaur R. Reviving mitochondrial bioenergetics: a relevant approach in epilepsy. Mitochondrion. 2021. https://doi.org/10.1016/j.mito.2021.03.009.
Han T, Qin Y, Mou C, Wang M, Jiang M, Liu B. Seizure induced synaptic plasticity alteration in hippocampus is mediated by IL-1β receptor through PI3K/Akt pathway. Am J Transl Res. 2016;8:4499.
Balosso S, Liu J, Bianchi ME, Vezzani A. Disulfide-containing high mobility group box-1 promotes N-methyl-d-aspartate receptor function and excitotoxicity by activating Toll-like receptor 4-dependent signaling in hippocampal neurons. Antioxid Redox Signal. 2014;21:1726–40. https://doi.org/10.1089/ars.2013.5349.
Paudel YN, Shaikh M, Chakraborti A, Kumari Y, Aledo-Serrano Á, Aleksovska K, Alvim MK, Othman I. HMGB1: a common biomarker and potential target for TBI, neuroinflammation, epilepsy, and cognitive dysfunction. Front Behav Neurosci. 2018;11(12):628. https://doi.org/10.3389/fnins.2018.00628.
Moran C, Sanz-Rodriguez A, Jimenez-Pacheco A, Martinez-Villareal J, McKiernan RC, Jimenez-Mateos EM, Mooney C, Woods I, Prehn JH, Henshall DC, Engel T. Bmf upregulation through the AMP-activated protein kinase pathway may protect the brain from seizure-induced cell death. Cell Death Differ. 2013;4:e606. https://doi.org/10.1038/cddis.2013.136.
Wu SB, Wu YT, Wu TP, Wei YH. Role of AMPK-mediated adaptive responses in human cells with mitochondrial dysfunction to oxidative stress. Biochimica Biochim Biophys Acta Gen Subj. 2014;1840:1331–44. https://doi.org/10.1016/j.bbagen.2013.10.034.
Kim SH, Yu HS, Park S, Park HG, Ahn YM, Kang UG, Kim YS. Electroconvulsive seizures induce autophagy by activating the AMPK signaling pathway in the rat frontal cortex. Int J Neuropsychopharmacol. 2020;23:42–52. https://doi.org/10.1093/ijnp/pyz055.
Peixoto CA, de Oliveira WH, da Racho Araújo SM, Nunes AK. AMPK activation: role in the signaling pathways of neuroinflammation and neurodegeneration. Exp Neurol. 2017;298:31–41. https://doi.org/10.1016/j.expneurol.2017.08.013.
Velagapudi R, El-Bakoush A, Lepiarz I, Ogunrinade F, Olajide OA. AMPK and SIRT1 activation contribute to inhibition of neuroinflammation by thymoquinone in BV2 microglia. Mol Cell Biochem. 2017;435:149–62. https://doi.org/10.1007/s11010-017-3064-3.
Zhang XM, Duan RS, Chen Z, Quezada HC, Mix E, Winblad B, Zhu J. IL-18 deficiency aggravates kainic acid-induced hippocampal neurodegeneration in C57BL/6 mice due to an overcompensation by IL-12. Exp Neurol. 2007;205:64–73. https://doi.org/10.1016/j.expneurol.2007.01.019.
Thawkar BS, Kaur G. Inhibitors of NF-κB and P2X7/NLRP3/Caspase 1 pathway in microglia: novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer’s disease. J Neuroimmunol. 2019;326:62–74. https://doi.org/10.1016/j.jneuroim.2018.11.010.
Wang BH, Hou Q, Lu YQ, Jia MM, Qiu T, Wang XH, Zhang ZX, Jiang Y. Ketogenic diet attenuates neuronal injury via autophagy and mitochondrial pathways in pentylenetetrazol-kindled seizures. Brain Res. 2018;1678:106–15. https://doi.org/10.1016/j.brainres.2017.10.009.
Liu Q, Zhang D, Hu D, Zhou X, Zhou Y. The role of mitochondria in NLRP3 inflammasome activation. Mol Immunol. 2018;103:115–24. https://doi.org/10.1016/j.molimm.2018.09.010.
Petrosillo G, Ruggiero FM, Pistolese M, Paradies G. Ca2+-induced ROS production promotes cytochrome c release from rat liver mitochondria via MPT-dependent and MPT-independent mechanisms. Role of cardiolipin . J Biol Chem. 2004. https://doi.org/10.1074/jbc.M407500200.
Perkins G, Bossy-Wetzel E, Ellisman MH. New insights into mitochondrial structure during cell death. Exp Neurol. 2009;218:183–92. https://doi.org/10.1016/j.expneurol.2009.05.021.
Chuang YC, Chen SD, Jou SB, Lin TK, Chen SF, Chen NC, Hsu CY. Sirtuin 1 regulates mitochondrial biogenesis and provides an endogenous neuroprotective mechanism against seizure-induced neuronal cell death in the hippocampus following status epilepticus. Int J Mol Sci. 2019;20:3588. https://doi.org/10.3390/ijms20143588.
Pallàs M, Casadesús G, Smith MA, Coto-Montes A, Pelegri C, Vilaplana J, Camins A. Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Curr Neurovasc Res. 2009;6:70–81. https://doi.org/10.2174/156720209787466019.
Javadov S, Kuznetsov A. Mitochondrial permeability transition and cell death: the role of cyclophilin d. Front physiol. 2013;4:76. https://doi.org/10.3389/fphys.2013.00076.
Grewal AK, Singh N, Singh TG. Neuroprotective effect of pharmacological postconditioning on cerebral ischaemia-reperfusion-induced injury in mice. J Pharm Pharmacol. 2019;71(6):956–70. https://doi.org/10.1111/jphp.13073.
Sun J, Gao X, Meng D, Xu Y, Wang X, Gu X, Guo M, Shao X, Yan H, Jiang C, Zheng Y. Antagomirs targeting mirorna-134 attenuates epilepsy in rats through regulation of oxidative stress, mitochondrial functions and autophagy. Front Pharmacol. 2017;8:524. https://doi.org/10.3389/fphar.2017.00524.
Chuang YC, Chen SD, Hsu CY, Chen SF, Chen NC, Jou SB. Resveratrol promotes mitochondrial biogenesis and protects against seizure-induced neuronal cell damage in the hippocampus following status epilepticus by activation of the PGC-1α signaling pathway Int J Mol Sci. 2019;20:998. https://doi.org/10.3390/ijms20040998.
Wang SJ, Zhao XH, Chen W, Bo N, Wang XJ, Chi ZF, Wu W. Sirtuin 1 activation enhances the PGC-1α/mitochondrial anti-oxidant system pathway in status epilepticus. Mol Med. 2015;11:521–6. https://doi.org/10.3892/mmr.2014.2724.
Sidorova-Darmos E, Sommer R, Eubanks JH. The role of SIRT3 in the brain under physiological and pathological conditions. Front Cell Neurosci. 2018;12:196. https://doi.org/10.3389/fncel.2018.00196.
Borowicz-Reutt KK, Czuczwar SJ. Role of oxidative stress in epileptogenesis and potential implications for therapy. Pharmacol Rep. 2020. https://doi.org/10.1007/s43440-020-00143-w.
Khan H, Tiwari P, Kaur A, Singh TG. Sirtuin acetylation and deacetylation: a complex paradigm in neurodegenerative disease. Mol Neurobiol. 2021;58(8):3903–17. https://doi.org/10.1007/s12035-021-02387-w.
Cho I, Jeong KH, Zhu J, Choi YH, Cho KH, Heo K, Kim WJ. Sirtuin3 protected against neuronal damage and cycled into nucleus in status epilepticus model. Mol Neurobiol. 2019;56:4894–903. https://doi.org/10.1007/s12035-018-1399-8.
Shimada T, Takemiya T, Sugiura H, Yamagata K. Role of inflammatory mediators in the pathogenesis of epilepsy. Mediat Inflamm. 2014. https://doi.org/10.1155/2014/901902.
Hixson KM, Cogswell M, Brooks-Kayal AR, Russek SJ. Evidence for a non-canonical JAK/STAT signaling pathway in the synthesis of the brain’s major ion channels and neurotransmitter receptors. BMC Genom. 2019;20:1–6. https://doi.org/10.1186/s12864-019-6033-2.
Nicolas CS, Amici M, Bortolotto ZA, Doherty A, Csaba Z, Fafouri A, Dournaud P, Gressens P, Collingridge GL, Peineau S. The role of JAK–STAT signaling within the CNS. JAK–STAT. 2013;2: e22925. https://doi.org/10.4161/jkst.22925.
Grabenstatter HL, Del Angel YC, Carlsen J, Wempe MF, White AM, Cogswell M, Russek SJ, Brooks-Kayal AR. The effect of STAT3 inhibition on status epilepticus and subsequent spontaneous seizures in the pilocarpine model of acquired epilepsy. Neurobiol Dis. 2014;62:73–85. https://doi.org/10.1016/j.nbd.2013.09.003.
Hokenson KE. BDNF signaling in epilepsy: TRKB-induced JAK/STAT pathway and phosphorylation of LSF in neurons (doctoral dissertation). Diss. https://hdl.handle.net/2144/16740. Accessed 2016.
Han CL, Liu YP, Guo CJ, Du TT, Jiang Y, Wang KL, Shao XQ, Meng FG, Zhang JG. The lncRNA H19 binding to let-7b promotes hippocampal glial cell activation and epileptic seizures by targeting Stat3 in a rat model of temporal lobe epilepsy. Cell Prolif. 2020;53: e12856. https://doi.org/10.1111/cpr.12856.
Vezzani A, Balosso S, Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol. 2019;15:459–72. https://doi.org/10.1038/s41582-019-0217-x.
Singh S, Singh TG. Role of nuclear factor kappa B (NF-κB) signalling in neurodegenerative diseases: an mechanistic approach. Curr Neuropharmacol. 2020;18(10):918–35. https://doi.org/10.2174/1570159X18666200207120949.
Kothur K, Bandodkar S, Wienholt L, Chu S, Pope A, Gill D, Dale RC. Etiology is the key determinant of neuroinflammation in epilepsy: elevation of cerebrospinal fluid cytokines and chemokines in febrile infection-related epilepsy syndrome and febrile status epilepticus. Epilepsia. 2019. https://doi.org/10.1111/epi.16275.
Granat L, Hunt RJ, Bateman JM. Mitochondrial retrograde signalling in neurological disease. Philos Trans R Soc B. 1801;2020(375):20190415. https://doi.org/10.1098/rstb.2019.0415.
Heuser K, Szokol K, Taubøll E. The role of glial cells in epilepsy. Tidsskr Nor Legeforen. 2014. https://doi.org/10.4045/tidsskr.12.1344.
Rehni AK, Singh TG. Modulation of leukotriene D4 attenuates the development of seizures in mice. Prostaglandins Leukot Essent Fatty Acids. 2011;85(2):97–106. https://doi.org/10.1016/j.plefa.2011.04.003.
Rehni AK, Singh TG, Singh N, Arora S. Tramadol-induced seizurogenic effect: a possible role of opioid-dependent histamine H1 receptor activation-linked mechanism. Naunyn Schmiedebergs Arch Pharmacol. 2010;381(1):11–9. https://doi.org/10.1007/s00210-009-0476-y.
Humphries F, Yang S, Wang B, Moynagh PN. RIP kinases: key decision makers in cell death and innate immunity. Cell Death Differ. 2015;22:225–36. https://doi.org/10.1038/cdd.2014.126.
Lin DQ, Cai XY, Wang CH, Yang B, Liang RS. Optimal concentration of necrostatin-1 for protecting against hippocampal neuronal damage in mice with status epilepticus. Neural Regen Res. 2020;15:936. https://doi.org/10.4103/1673-5374.268903.
Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab. 2010. https://doi.org/10.1152/ajpendo.00745.2009.
Rousset CI, Leiper FC, Kichev A, Gressens P, Carling D, Hagberg H, Thornton C. A dual role for AMP-activated protein kinase (AMPK) during neonatal hypoxic–ischaemic brain injury in mice. J Neurochem. 2015;133:242–52. https://doi.org/10.1111/jnc.13034.
Jiang S, Li T, Ji T, Yi W, Yang Z, Wang S, Yang Y, Gu C. AMPK: potential therapeutic target for ischemic stroke. Theranostics. 2018;8:4535. https://doi.org/10.7150/thno.25674.
Ronnett GV, Ramamurthy S, Kleman AM, Landree LE, Aja S. AMPK in the brain: its roles in energy balance and neuroprotection. J Neurochem. 2009;109:17–23. https://doi.org/10.1111/j.1471-4159.2009.05916.x.
Waldbaum S, Patel M. Mitochondrial dysfunction and oxidative stress: a contributing link to acquired epilepsy? J Bioenerg Biomembr. 2010;42:449–55. https://doi.org/10.1007/s10863-010-9320-9.
Xu W, Yan J, Ocak U, Lenahan C, Shao A, Tang J, Zhang J, Zhang JH. Melanocortin 1 receptor attenuates early brain injury following subarachnoid hemorrhage by controlling mitochondrial metabolism via AMPK/SIRT1/PGC-1α pathway in rats. Theranostics. 2021;11:522. https://doi.org/10.7150/thno.49426.
Bernardo A, Minghetti L. PPAR-γ agonists as regulators of microglial activation and brain inflammation. Curr Pharm Des. 2006;12:93–109. https://doi.org/10.2174/138161206780574579.
Wilson G, Bryan J, Cranston K, Kitzes J, Nederbragt L, Teal TK. Good enough practices in scientific computing. PLoS Comput Biol. 2017;13: e1005510. https://doi.org/10.1371/journal.pone.0144806.
Villapol S. Roles of peroxisome proliferator-activated receptor gamma on brain and peripheral inflammation. Cell Mol Neurobiol. 2018;38:121–32. https://doi.org/10.1007/s10571-017-0554-5.
Meng QQ, Feng ZC, Zhang XL, Hu LQ, Wang M, Zhang HF, Li SM. PPAR-γ activation exerts an anti-inflammatory effect by suppressing the nlrp3 inflammasome in spinal cord-derived neurons. Mediat Inflamm. 2019. https://doi.org/10.1155/2019/6386729.
Chuang YC, Lin TK, Huang HY, Chang WN, Liou CW, Chen SD, Chang AY, Chan SH. Peroxisome proliferator-activated receptors γ/mitochondrial uncoupling protein 2 signaling protects against seizure-induced neuronal cell death in the hippocampus following experimental status epilepticus. J Neuroinflamm. 2012. https://doi.org/10.1186/1742-2094-9-184.
Abdallah DM. Anticonvulsant potential of the peroxisome proliferator-activated receptor gamma agonist pioglitazone in pentylenetetrazole-induced acute seizures and kindling in mice. Brain Res. 2010;1351:246–53. https://doi.org/10.1016/j.brainres.2010.06.034.
Lucchi C, Costa AM, Giordano C, Curia G, Piat M, Leo G, Vinet J, Brunel L, Fehrentz JA, Martinez J, Torsello A. Involvement of PPARγ in the anticonvulsant activity of EP-80317, a ghrelin receptor antagonist. Front Pharmacol. 2017;8:676. https://doi.org/10.3389/fphar.2017.00676.
Hung TY, Chu FL, Wu DC, Wu SN, Huang CW. The protective role of peroxisome proliferator-activated receptor-gamma in seizure and neuronal excitotoxicity. Mol Neurobiol. 2019;56:5497–506. https://doi.org/10.1007/s12035-018-1457-2.
Saha L, Bhandari S, Bhatia A, Banerjee D, Chakrabarti A. Anti-kindling effect of bezafibrate, a peroxisome proliferator-activated receptors alpha agonist, in pentylenetetrazole induced kindling seizure model. J Epilepsy Res. 2014;4:45. https://doi.org/10.14581/jer.14011.
Huang C, Chi XS, Li R, Hu X, Xu HX, Li JM, Zhou D. Inhibition of P2X7 receptor ameliorates nuclear factor-kappa B mediated neuroinflammation induced by status epilepticus in rat hippocampus. J Mol Neurosci. 2017;63:173–84. https://doi.org/10.1007/s12031-017-0968-z.
Liang X, Samways DS, Wolf K, Bowles EA, Richards JP, Bruno J, Dutertre S, DiPaolo RJ, Egan TM. Quantifying Ca2+ current and permeability in ATP-gated P2X7 receptors. J Biol Chem. 2015;290:7930–42. https://doi.org/10.1074/jbc.M114.627810.
Giorgi C, Agnoletto C, Bononi A, Bonora M, De Marchi E, Marchi S, Missiroli S, Patergnani S, Poletti F, Rimessi A, Suski JM. Mitochondrial calcium homeostasis as potential target for mitochondrial medicine. Mitochondrion. 2012;12:77–85. https://doi.org/10.1016/j.mito.2011.07.004.
Kent AC, El Baradie KB, Hamrick MW. Targeting the mitochondrial permeability transition pore to prevent age-associated cell damage and neurodegeneration. Oxid Med Cell Longev. 2021. https://doi.org/10.1155/2021/6626484.
Zhang Z, Sun T, Niu JG, He ZQ, Liu Y, Wang F. Amentoflavone protects hippocampal neurons: anti-inflammatory, antioxidative, and antiapoptotic effects. Neural Regen Res. 2015;10:1125. https://doi.org/10.4103/1673-5374.160109.
Panigrahy SR, Pradhan S, Maharana CS. Amelioration of oxidative stress and neuroinflammation by Saroglitazar, a dual PPARα/γ agonist in MES induced epileptic rats. Biomed Pharmacol J. 2019;12:1985–91. https://doi.org/10.13005/bpj/1830.
Tambe R, Jain P, Patil S, Ghumatkar P, Sathaye S. Antiepileptogenic effects of borneol in pentylenetetrazole-induced kindling in mice. Naunyn-Schmiedeberg’s Arch Pharmacol. 2016;389:467–75. https://doi.org/10.1007/s00210-016-1220-z.
Shu Y, Zhu C, Zeng M, Zhan Q, Hu Z, Wu X. The protective effect of carbenoxolone on gap junction damage in the hippocampal CA1 area of a temporal lobe epilepsy rat model. Ann Transl Med. 2019. https://doi.org/10.21037/atm.2019.11.04.
Hino H, Takahashi H, Suzuki Y, Tanaka J, Ishii E, Fukuda M. Anticonvulsive effect of paeoniflorin on experimental febrile seizures in immature rats: possible application for febrile seizures in children. PLoS ONE. 2012;7: e42920. https://doi.org/10.1371/journal.pone.0042920.
Chen YF, Lee MM, Fang HL, Yang JG, Chen YC, Tsai HY. Paeoniflorin inhibits excitatory amino acid agonist-and high-dose morphine-induced nociceptive behavior in mice via modulation of N-methyl-d-aspartate receptors. BMC Complement Altern Med. 2016;16:1–4. https://doi.org/10.1186/s12906-016-1230-x.
Hu PF, Chen WP, Bao JP, Wu LD. Paeoniflorin inhibits IL-1β-induced chondrocyte apoptosis by regulating the Bax/Bcl-2/caspase-3 signaling pathway. Mol Med. 2018;17:6194–200. https://doi.org/10.3892/mmr.2018.8631.
Cong C, Kluwe L, Li S, Liu X, Liu Y, Liu H, Gui W, Liu T, Xu L. Paeoniflorin inhibits tributyltin chloride-induced apoptosis in hypothalamic neurons via inhibition of MKK4-JNK signaling pathway. J Ethnopharmacol. 2019;237:1–8. https://doi.org/10.1016/j.jep.2019.03.030.
Singh N, Saha L, Kumari P, Singh J, Bhatia A, Banerjee D, Chakrabarti A. Effect of dimethyl fumarate on neuroinflammation and apoptosis in pentylenetetrazol kindling model in rats. Brain Res Bull. 2019;144:233–45. https://doi.org/10.1016/j.brainresbull.2018.11.013.
Kahn-Kirby AH, Amagata A, Maeder CI, Mei JJ, Sideris S, Kosaka Y, Hinman A, Malone SA, Bruegger JJ, Wang L, Kim V. Targeting ferroptosis: a novel therapeutic strategy for the treatment of mitochondrial disease-related epilepsy. PLoS ONE. 2019;14: e0214250. https://doi.org/10.1371/journal.pone.0214250.
Elsayed AA, Menze ET, Tadros MG, Ibrahim BM, Sabri NA, Khalifa AE. Effects of genistein on pentylenetetrazole-induced behavioral and neurochemical deficits in ovariectomized rats. Naunyn-Schmiedeberg’s Arch Pharmacol. 2018;391:27–36. https://doi.org/10.1007/s00210-017-1435-7.
Jiang G, Wu H, Hu Y, Li J, Li Q. Gastrodin inhibits glutamate-induced apoptosis of PC12 cells via inhibition of CaMKII/ASK-1/p38 MAPK/p53 signaling cascade. Cell Mol Neurobiol. 2014;34:591–602. https://doi.org/10.1007/s10571-014-0043-z.
Chen L, Liu X, Wang H, Qu M. Gastrodin attenuates pentylenetetrazole-induced seizures by modulating the mitogen-activated protein kinase-associated inflammatory responses in mice. Neurosci Bull. 2017;33:264–72. https://doi.org/10.1007/s12264-016-0084-z.
Yamamoto HA, Mohanan PV. Ganglioside GT1B and melatonin inhibit brain mitochondrial DNA damage and seizures induced by kainic acid in mice. Brain Res. 2003;964:100–6. https://doi.org/10.1016/S0006-8993(02)04083-0.
Yang N, Guan QW, Chen FH, Xia QX, Yin XX, Zhou HH, Mao XY. Antioxidants targeting mitochondrial oxidative stress: promising neuroprotectants for epilepsy. Oxid Med Cell Longev. 2020. https://doi.org/10.1155/2020/6687185.
Hellmich HL, Rojo DR, Micci MA, Sell SL, Boone DR, Crookshanks JM, DeWitt DS, Masel BE, Prough DS. Pathway analysis reveals common pro-survival mechanisms of metyrapone and carbenoxolone after traumatic brain injury. PLoS ONE. 2013;8: e53230. https://doi.org/10.1371/journal.pone.0053230.
García-García L, Shiha AA, de la Rosa RF, Delgado M, Silván Á, Bascuñana P, Bankstahl JP, Gomez F, Pozo MA. Metyrapone prevents brain damage induced by status epilepticus in the rat lithium-pilocarpine model. Neuropharmacology. 2017;123:261–73. https://doi.org/10.1016/j.neuropharm.2017.05.007.
Reddy SD, Younus I, Sridhar V, Reddy DS. Neuroimaging biomarkers of experimental epileptogenesis and refractory epilepsy. Int J Mol Sci. 2019;20:220. https://doi.org/10.3390/ijms20010220.
Castro OW, Upadhya D, Kodali M, Shetty AK. Resveratrol for easing status epilepticus induced brain injury, inflammation, epileptogenesis, and cognitive and memory dysfunction—are we there yet? Front Neurol. 2017;8:603. https://doi.org/10.3389/fneur.2017.00603.
Gao X, Zhao XL, Zhu YH, Li XM, Xu Q, Lin HD, Wang MW. Tetramethylpyrazine protects palmitate-induced oxidative damage and mitochondrial dysfunction in C2C12 myotubes. Life Sci. 2011;88:803–9. https://doi.org/10.1016/j.lfs.2011.02.025.
Jin Y, Cai S, Jiang Y, Zhong K, Wen C, Ruan Y, Chew LA, Khanna R, Xu Z, Yu J. Tetramethylpyrazine reduces epileptogenesis progression in electrical kindling models by modulating hippocampal excitatory neurotransmission. ACS Chem Neurosci. 2019;10:4854–63. https://doi.org/10.1021/acschemneuro.9b00575.
Grewal AK, Singh TG, Sharma D, Sharma V, Singh M, Rahman MH, Najda A, Walasek-Janusz M, Kamel M, Albadrani GM, Akhtar MF, Saleem A, Abdel-Daim MM. Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed Pharmacother. 2021;140: 111729. https://doi.org/10.1016/j.biopha.2021.111729.
Acknowledgements
The authors are grateful to the Chitkara College of Pharmacy, Chitkara University, Rajpura, Patiala, Punjab, India for providing the necessary facilities to carry out the research work.
Funding
Nil.
Author information
Authors and Affiliations
Contributions
Conceptualization: conceived and designed the experiments: TGS. Analyzed the data: SS and TGS. Wrote the manuscript: SS. Editing of the manuscript: TGS; critically reviewed the article: TGS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, S., Singh, T.G. Emerging perspectives on mitochondrial dysfunctioning and inflammation in epileptogenesis. Inflamm. Res. 70, 1027–1042 (2021). https://doi.org/10.1007/s00011-021-01511-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-021-01511-9