Abstract
Purpose
Ouabain, an Na+/K+-ATPase inhibitor hormone, presents immunomodulatory actions, including anti-inflammatory effect on acute inflammation models.
Methods
In the present study, the effect of ouabain in a model of allergic airway inflammation induced by ovalbumin (OVA) was assessed.
Results
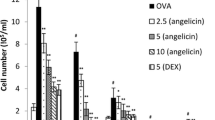
Initially, it was observed that ouabain treatment inhibited cellular migration induced by OVA on bronchoalveolar lavage fluid (BALF), mostly granulocytes, without modulating macrophage migration. In addition, it was observed, by flow cytometry, that ouabain reduces CD3high lymphocytes cells on BALF. Furthermore, treatment with ouabain decreased IL-4 and IL-13 levels on BALF. Ouabain also promoted pulmonary histological alterations, including decreased cell migration into peribronchiolar and perivascular areas, and reduced mucus production in bronchioles regions observed through hematoxylin–eosin (HE) and by periodic acid-Schiff stain, respectively. Allergic airway inflammation is characterized by high OVA-specific IgE serum titer. This parameter was also reduced by the treatment with ouabain.
Conclusions
Therefore, our data demonstrate that ouabain negatively modulates allergic airway inflammation induced by OVA.







Similar content being viewed by others
Change history
16 January 2018
In the original publication, author missed to include the financial support from CAPES/PROCAD-2013. The complete funding text should read as follows.
References
Schoner W. Endogenous cardiac glycosides, a new class of steroid hormones. Eur J Biochem. 2002;269:2440–8. doi:10.1046/j.1432-1033.2002.02911.x.
Erdmann E, Schoner W. Ouabain-receptor interactions in (Na+K+)-ATPase preparations from different tissues and species determination of kinetic constants and dissociation constants. BBA Biomembr. 1973;307:386–98. doi:10.1007/BF00501108.
Bagrov AY, Roukoyatkina NI, Pinaev AG, Dmitrieva RI, Fedorova OV. Effects of two endogenous Na+, K+-ATPase inhibitors, marinobufagenin and ouabain, on isolated rat aorta. Eur J Pharmacol. 1995;274:151–8. doi:10.1016/0014-2999(94)00735-P.
Hamlyn JM, Blaustein MP, Bova S, DuCharme DW, Harris DW, Mandel F, et al. Identification and characterization of a ouabain-like compound from human plasma. Proc Natl Acad Sci USA. 1991;88:6259–63. doi:10.1073/pnas.88.21.9907-d.
Ferrandi M, Manunta P, Balzan S, Hamlyn JM, Bianchi G, Ferrari P. Ouabain-like factor quantification in mammalian tissues and plasma: comparison of two independent assays. Hypertension. 1997;30:886–96. doi:10.1161/01.HYP.30.4.886.
Schoner W, Bauer N, Müller-Ehmsen J, Krämer U, Hambarchian N, Schwinger R, et al. Ouabain as a mammalian hormone. Ann N Y Acad Sci. 2003;986:678–84. doi:10.1111/j.1749-6632.2003.tb07282.x.
Bagrov AY, Shapiro JI. Endogenous digitalis: pathophysiologic roles and therapeutic applications. Nat Clin Pract Nephrol. 2008;4:378–92. doi:10.1038/ncpneph0848.
Tymiak AA, Norman JA, Bolgar M, DiDonato GC, Lee H, Parker WL, et al. Physicochemical characterization of a ouabain isomer isolated from bovine hypothalamus. Proc Natl Acad Sci USA. 1993;90:8189–93.
Kawamura A, Guo J, Itagaki Y, Bell C, Wang Y, Haupert GT, et al. On the structure of endogenous ouabain. Proc Natl Acad Sci USA. 1999;96:6654–9. doi:10.1073/pnas.96.12.6654.
Schoner W. Ouabain, a new steroid hormone of adrenal gland and hypothalamus. Exp Clin Endocrinol Diabetes. 2000;108:449–54. doi:10.1055/s-2000-8140.
Goto A, Yamada K, Nagoshi H, Terano Y, Omata M. Stress-induced elevation of ouabain like compound in rat plasma and adrenal. Hypertension. 1995;26:1173–6. doi:10.1161/01.HYP.26.6.1173.
Bauer N, Müller-Ehmsen J, Krämer U, Hambarchian N, Zobel C, Schwinger RH, et al. Ouabain-like compound changes rapidly on physical exercise in humans and dogs: effects of β-blockade and angiotensin-converting enzyme inhibition. Hypertension. 2005;45:1024–8. doi:10.1161/01.HYP.0000165024.47728.f7.
Quastel MR, Kaplan JG. Inhibition by ouabain of human lymphocyte transformation induced by phytohaemagglutinin in vitro. Nature. 1968;219:198–200. doi:10.1038/219198a0.
Olej B, de La Rocque L, Castilho FP, Mediano IF, Campos MM, Rumjanek VM. Effect of ouabain on lymphokine-activated killer cells. Int J Immunopharmacol. 1994;16:769–74. doi:10.1016/0192-0561(94)90097-3.
Olej B, dos Santos NF, Leal L, Rumjanek VM. Ouabain induces apoptosis on PHA-activated lymphocytes. Biosci Rep. 1998;18:1–7. doi:10.1023/A:1022259832207.
Szamel M, Schneider S, Resch K. Functional interrelationship between Na+/K+-ATPase and lysolecithin acyltransferase in plasma membranes of mitogen-stimulated rabbit thymocytes. J Biol Chem. 1981;256:9198–204.
Rodrigues-Mascarenhas S, Bloise FF, Moscat J, Rumjanek VM. Ouabain inhibits p38 activation in thymocytes. Cell Biol Int. 2008;32:1323–8. doi:10.1016/j.cellbi.2008.07.012.
Valente RC, Nascimento CR, Araujo EG, Rumjanek VM. mCD14 expression in human monocytes is downregulated by ouabain via transactivation of epithelial growth factor receptor and activation of p38 mitogen-activated protein kinase. Neuroimmunomodulation. 2009;16:228–36. doi:10.1159/000212383.
Teixeira MP, Rumjanek VM. Ouabain affects the expression of activation markers, cytokine production, and endocytosis of human monocytes. Mediat Inflamm. 2014. doi:10.1155/2014/760368.
Nascimento CR, Valente RC, Echevarria-Lima J, Fontes CF, de Araujo-Martins L, Araujo EG, et al. The influence of ouabain on human dendritic cells maturation. Mediat Inflamm. 2014. doi:10.1155/2014/494956.
Rodrigues-Mascarenhas S, Echevarria-Lima J, Fernandes dos Santos N, Rumjanek VM. CD69 expression induced by thapsigargin, phorbol ester and ouabain on thymocytes is dependent on external Ca2+ entry. Life Sci. 2003;73:1037–51. doi:10.1016/S0024-3205(03)00377-1.
Rodrigues-Mascarenhas S, dos Santos NF, Rumjanek VM. Synergistic effect between ouabain and glucocorticoids for the induction of thymic atrophy. Biosci Rep. 2006;26:159–69. doi:10.1007/s10540-006-9012-1.
Da Silva JMC, das Neves Azevedo A, dos Santos Barbosa RP, Vianna TA, Fittipaldi J, Teixeira MP, et al. Dynamics of murine B lymphocytes is modulated by in vivo treatment with steroid ouabain. Immunobiology. 2016;221:368–76. doi:10.1016/j.imbio.2015.09.020.
De Vasconcelos DI, Leite JA, Carneiro LT, Piuvezam MR, de Lima MR, de Morais LC, et al. Anti-inflammatory and antinociceptive activity of ouabain in mice. Mediat Inflamm. 2011. doi:10.1155/2011/912925.
Leite JA, Alves AK, Galvão JGFM, Teixeira MP, Cavalcante-Silva LHA, Scavone C, et al. Ouabain modulates zymosan-induced peritonitis in mice. Mediat Inflamm. 2015. doi:10.1155/2015/265798.
Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35. doi:10.1038/nature07201.
Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16:45–56. doi:10.1038/ni.3049.
De Monchy JGR, Kauffman HF, Venge P, Koëter GH, Jansen HM, Sluiter HJ, et al. Bronchoalveolar eosinophilia during allergen-induced late asthmatic reactions. Am Rev Respir Dis. 1985;131:373–6. doi:10.1164/arrd.1985.131.3.373.
Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–95. doi:10.1164/rccm.200903-0392OC.
Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, et al. Predominant Th2-like bronchoalveolar T lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi:10.1056/NEJM199201303260504.
Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci USA. 2007;104:15858–63. doi:10.1073/pnas.0707413104.
Cheng D, Xue Z, Yi L, Shi H, Zhang K, Huo X, et al. Epithelial interleukin-25 is a key mediator in Th2-high, corticosteroid-responsive asthma. Am J Respir Crit Care Med. 2014;190:639–48. doi:10.1164/rccm.201403-0505OC.
Urwin DL, Schwenger GT, Groth DM, Sanderson CJ. Distal regulatory elements play an important role in regulation of the human IL-5 gene. Eur J Immunol. 2004;34:3633–43. doi:10.1002/eji.200425279.
Agrawal KP, Reed CE, Hyatt RE, Imber WE, Krell WS. Airway responses to inhaled ouabain in subjects with and without asthma. Mayo Clin Proc. 1986;61:778–84. doi:10.1016/S0025-6196(12)64816-2.
Senol M, Ozerol IH, Patel AV, Skoner DP. The effect of Na+–K+ ATPase inhibition by ouabain on histamine release from human cutaneous mast cells. Mol Cell Biochem. 2007;294:25–9. doi:10.1007/s11010-006-9180-0.
Lago J, Alfonso A, Vieytes MR, Botana LM. Ouabain-induced enhancement of rat mast cells response. Modulation by protein phosphorylation and intracellular pH. Cell Signal. 2001;13:515–24. doi:10.1016/S0898-6568(01)00169-3.
Vieira GC, De Lima JF, De Figueiredo RC, Mascarenhas SR, Bezerra-Santos CR, Piuvezam MR. Inhaled Cissampelos sympodialis down-regulates airway allergic reaction by reducing lung CD3+ T cells. Phytother Res. 2012;27:916–25. doi:10.1002/ptr.4791.
Holt PG, Rose AH, Batty JE, Turner KJ. Introduction of adjuvant-independent IgE responses in inbred mice: primary, secondary and persistent IgE responses to ovalbumin and ovomucoid. Int Arch Allergy Appl Immunol. 1981;65:42–50. doi:10.1159/000232736.
Jacob PL, Leite JA, Alves AK, Rodrigues YK, Amorim FM, Néris PL, et al. Immunomodulatory activity of ouabain in Leishmania leishmania amazonensis infected Swiss mice. Parasitol Res. 2013;112:1313–21. doi:10.1007/s00436-012-3146-9.
Venkayya R, Lam M, Willkom M, Grünig G, Corry DB, Erle DJ. The Th2 lymphocyte products IL-4 and IL-13 rapidly induce airway hyperresponsiveness through direct effects on resident airway cells. Am J Respir Cell Mol Biol. 2002;26:202–8. doi:10.1165/ajrcmb.26.2.4600.
Deo SS, Mistry KJ, Kakade AM, Niphadkar PV. Role played by Th2 type cytokines in IgE mediated allergy and asthma. Lung India. 2010;27:66–71. doi:10.4103/0970-2113.63609.
Cowden JM, Riley JP, Ma JY, Thurmond RL, Dunford PJ. Histamine H4 receptor antagonism diminishes existing airway inflammation and dysfunction via modulation of Th2 cytokines. Respir Res. 2010;11:1–12. doi:10.1186/1465-9921-11-86.
Van Rijt LS, Vos N, Hijdra D, de Vries VC, Hoogsteden HC, Lambrecht BN. Airway eosinophils accumulate in the mediastinal lymph nodes but lack antigen-presenting potential for naive T cells. J Immunol. 2003;171:3372–8. doi:10.4049/jimmunol.171.7.3372.
Yousefi S, Simon D, Simon HU. Eosinophil extracellular DNA traps: molecular mechanisms and potential roles in disease. Curr Opin Immunol. 2012;24:736–9. doi:10.1016/j.coi.2012.08.010.
Ninsontia C, Chanvorachote P. Ouabain mediates integrin switch in human lung cancer cells. Anticancer Res. 2014;34:5495–502.
Takada Y, Matsuo K, Ogura H, Bai L, Toki A, Wang L, et al. Odoroside A and ouabain inhibit Na+/K+-ATPase and prevent NF-kappaB-inducible protein expression by blocking Na+-dependent amino acid transport. Biochem Pharmacol. 2009;78:1157–66. doi:10.1016/j.bcp.2009.06.027.
Pongrakhananon V, Chunhacha P, Chanvorachote P. Ouabain suppresses the migratory behavior of lung cancer cells. PLoS One. 2013;8:e68623. doi:10.1371/journal.pone.0068623.
Shin HK, Ryu BJ, Choi SW, Kim SH, Lee K. Inactivation of Src-to-ezrin pathway: a possible mechanism in the ouabain-mediated inhibition of A549 cell migration. Biomed Res Int. 2015;2015:537136. doi:10.1155/2015/537136.
Gonçalves-de-Albuquerque CF, Burth P, Silva AR, de Moraes IM, Oliveira FM, Santelli RE, et al. Murine lung injury caused by Leptospira interrogans glycolipoprotein, a specific Na/K-ATPase inhibitor. Respir Res. 2014;15:1–12. doi:10.1186/s12931-014-0093-2.
Azzawi M, Johnston PW, Majumdar S, Kay AB, Jeffery PK. T lymphocytes and activated eosinophils in airway mucosa in fatal asthma and cystic fibrosis. Am Rev Respir Dis. 1992;145:1477–82.
Bystrom J, Amin K, Bishop-Bailey D. Analysing the eosinophil cationic protein—a clue to the function of the eosinophil granulocyte. Respir Res. 2011;12:1465–9921.
Amin K. The role of mast cells in allergic inflammation. Respir Med. 2012;106:9–14.
Carlson M, Peterson C, Venge P. The influence of IL-3, IL-5, and GM-CSF on normal human eosinophil and neutrophil C3b induced degranulation. Allergy. 1993;48:437–42.
Wilson JW, Djukanović R, Howarth PH, Holgate ST. Lymphocyte Activation in bronchoalveolar lavage and peripheral blood in atopic asthma. Am Rev Respir Dis. 1992;4:958–60. doi:10.1164/ajrccm/145.4_Pt_1.958.
Slager RE, Li H, Moore W, Hawkins G, Peters SP, Busse WW, et al. Predictive model of severe atopic asthma phenotypes using interleukin-4/13 pathway polymorphisms. Am J Respir Crit Care Med. 2011;183:1332. doi:10.1164/ajrccm-conference.2011.183.1_MeetingAbstracts.A1332.
Mattila PS, Ullman KS, Fiering S, et al. The action of cyclosporin A and FK506 suggest a novel step in the activation of T lymphocytes. EMBO J. 1990;9:4425–33.
Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–47.
Diehl S, Krahl T, Rinaldi L, Norton R, Irvin GC, Rincón M. Inhibition of NFAT specifically in T cells prevents allergic pulmonary inflammation. J Immunol. 2004;172:3597–603. doi:10.4049/jimmunol.172.6.3597.
Brusselle GG, Kips JC, Tavernier JH, van der Heyden JG, Cuvelier CA, Pauwels RA, et al. Attenuation of allergic airway inflammation in IL-4 deficient mice. Clin Exp Allergy. 1994;24:73–80. doi:10.1111/j.1365-2222.1994.tb00920.x.
Corry DB, Folkesson HG, Warnock ML, Erle DJ, Matthay MA, Wiener-Kronish JP, et al. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996;183:109–17. doi:10.1084/jem.183.1.109.
Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi:10.1007/s11882-004-0057-6.
Rosenstein RK, Bezbradica JS, Yu S, Medzhitov R. Signaling pathways activated by a protease allergen in basophils. Proc Natl Acad Sci USA. 2014;111:E4963–71. doi:10.1073/pnas.1418959111.
Liew FY. T(H)1 and T(H)2 cells: a historical perspective. Nat Rev Immunol. 2002;2:55–60. doi:10.1038/nri705.
Huang TJ, MacAry P, Eynott P, et al. Allergen-specific Th1 cells counteract effecrent Th2 cell-dependent bronchial hyperresponsiveness and eosinophilic inflammation, partly via IFN-g. J Immunol. 2001;166:207–17.
Cohn L, Homer RJ, Niu N, Bottomly K. T helper 1 cells and interferon gamma regulate allergic airway inflammation and mucus production. J Exp Med. 1999;190:1309–18.
Boumpas DT, Chrousos GP, Wilder RL, Cupps TR, Balow JE. Glucocorticoid therapy for immune-mediated diseases: basic and clinical correlates. Ann Intern Med. 1993;119:1198.
Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17:233–47. doi:10.1038/nri.2017.1.
De Bosscher K, Haegeman G. Minireview: latest perspectives on anti-inflammatory actions of glucocorticoids. Mol Endocrinol. 2008;23:281–91.
Wang D, Müller N, McPherson KG, Reichardt HM. Glucocorticoids engage different signal transduction pathways to induce apoptosis in thymocytes and mature T cells. J. Immunol. 2006;176:1695–702.
Franchimont D, Galon J, Gadina M, Visconti R, Zhou Y, Aringer M, et al. Inhibition of Th1 immune response by glucocorticoids: dexamethasone selectively inhibits IL-12-induced Stat4 phosphorylation in T lymphocytes. J. Immunol. 2000;164:1768–74.
Barnes PJ. Th2 cytokines and asthma: an introduction. Respir Res. 2001;2:64–5.
Paiva LS, Costa KM, Canto FB, Cabral VR, Fucs R, Nobrega A, et al. Modulation of mature B cells in mice following treatment with ouabain. Immunobiology. 2011;216:1038–43. doi:10.1016/j.imbio.2011.03.002.
Geha RS, Jabara HH, Brodeur SR. The regulation of immunoglobulin E class-switch recombination. Nat Rev Immunol. 2003;3:721–32. doi:10.1038/nri1181.
Author information
Authors and Affiliations
Contributions
JGFMG and SRM: conceived and designed the experiments. JGFMG, DCMC, LKDPF, and LAMPF performed experimental model of allergic airway inflammation; JGFMG and LHACS performed ELISA and flow cytometry experiments; JGFMG and TMM performed dosage of IgE; JGFMG, AFA, and FAAFG performed lung histology; JGFMG, LHACS, MRP, and SRM analyzed the data and wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by CNPq (“Conselho Nacional de Desenvolvimento Científico e Tecnológico”, Grant Number 478536/2013-5) and “INCT para Controle do Câncer”.
Additional information
Responsible Editor: Bernhard Gibbs.
A correction to this article is available online at https://doi.org/10.1007/s00011-018-1130-2.
Rights and permissions
About this article
Cite this article
Galvão, J.G.F.M., Cavalcante-Silva, L.H.A., Carvalho, D.C.M. et al. Ouabain attenuates ovalbumin-induced airway inflammation. Inflamm. Res. 66, 1117–1130 (2017). https://doi.org/10.1007/s00011-017-1092-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-017-1092-9