Abstract
Objective and design
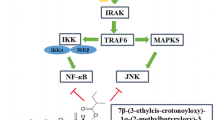
The purpose of this study was to evaluate the anti-inflammatory and anti-arthritic activities of 3,4-dihydro-2,2-dimethyl-2H-naphthol[1,2-b]pyran-5,6-dione (β-lapachone; β-lap) and to elucidate its probable mode of action.
Methods
Carrageenan-induced paw edema, cell migration evaluation and production of pro-inflammatory cytokines tumor necrosis factor (TNF)-α, interleukin (IL)-6 and nitric oxide were used for this study. Freund’s complete adjuvant (FCA)-induced arthritis was used as a model of chronic inflammation. β-Lap was tested in doses of 40 and 60 mg/kg, orally.
Results
In the paw edema test, the dose of 60 mg/kg gave a higher percentage inhibition of edema (49.3 %) than control. β-Lap inhibited neutrophil migration and reduced concentrations of TNF-α, IL-6 and NO in peritoneal exudates of animals with peritonitis. In the arthritis test, β-lap inhibited edema and NO production in the serum of treated animals.
Conclusion
Significant anti-inflammatory and anti-arthritic activities were observed in animals treated with β-lap. The effects of β-lap can be attributed in part to immunomodulation with reduction of pro-inflammatory cytokines and NO.


Similar content being viewed by others
References
Moon DO, Kang CH, Kim MO, Jeon YJ, Lee JD, Choi YH, Kim GY. β-Lapachone (LAPA) decreases cell viability and telomerase activity in leukemia cells: suppression of telomerase activity by LAPA. J Med Food. 2010;13:481–8.
Ferreira SB, Salomão K, Silva FC, Pinto AV, Kaiser CR, Pinto AC, Ferreira VF, Castro SI. Synthesis and anti-Trypanosoma cruzi activity of β-lapachone analogues. Eur J Med Chem. 2011;46:3071–7.
De Almeida ER, da Silva Filho AA, dos Santos ER, Lopes CA. Anti-inflammatory action of lapachol. J Ethnopharmacol. 1990;29:239–41.
Tzeng HP, Ho FM, Chao KF, Kuo ML, Lin-Shiau SY, Liu SH. Beta-Lapachone reduces endotoxin-induced macrophage activation and lung edema and mortality. Am J Respir Crit Care Med. 2003;168:85–91.
Guiraud P, Steiman R, Campos-Takaki GM, Seigle-Murandi F, Simeon de BM. Comparison of antibacterial and antifungal activities of lapachol and beta-lapachone. Planta Med. 1994;60:373–4.
Pereira EM. Machado Tde B, Leal IC, Jesus DM, Damaso CR, Pinto AV, Giambiagi-de Marval M, Kuster RM, Santos KR. Tabebuia avellanedae naphthoquinones: activity against methicillin-resistant staphylococcal strains, cytotoxic activity and in vivo dermal irritability analysis. Ann Clin Microbiol Antimicrob. 2006;22(5):5.
Oliveira RA, Azevedo-Ximenes E, Luzzati R, Garcia RC. The hydroxy-naphthoquinone lapachol arrests mycobacterial growth and immunomodulates host macrophages. Int Immunopharmacol. 2010;10:1463–73.
Li CJ, Zhang LJ, Dezube BJ, Crumpacker CS, Pardee AB. Three inhibitors of type 1 human immunodeficiency virus long terminal repeat-directed gene expression and virus replication. Proc Natl Acad Sci USA. 1993;90:1839–42.
Kung HN, Yang MJ, Chang CF, Chau YP, Lu KS. In vitro and in vivo wound healing-promoting activities of β-lapachone. Am J Physiol Cell Physiol. 2008;295:931–43.
Schuerch AR, Wehrli W. Beta-Lapachone, an inhibitor of oncornavirus reverse transcriptase and eukaryotic DNA polymerase-alpha. Inhibitory effect, thiol dependence and specificity. Eur J Biochem. 1978;84:197–205.
Boorstein RJ, Pardee AB. Coordinate inhibition of DNA synthesis and thymidylate synthase activity following DNA damage and repair. Biochem Biophys Res Commun. 1983;117:30–6.
Li YZ, Li CJ, Pinto AV, Pardee AB. Release of mitochondrial cytochrome c in both apoptosis and necrosis induced by beta-lapachone in human carcinoma cells. Mol Med. 1999;5:232–9.
Chau YP, Shiah SG, Don MJ, Kuo ML. Involvement of hydrogen peroxide in topoisomerase inhibitor b-lapachone-induced apoptosis and differentiation in human leukemia cells. Free Radic Biol Med. 1998;24:660–70.
Li CJ, Averboukh L, Pardee AB. Beta-lapachone, a novel DNA topoisomerase I inhibitor with a mode of action different from camptothecin. J Biol Chem. 1993;268:22463–8.
Krishnan P, Bastow KF. Novel mechanism of cellular DNA topoisomerase II inhibition by the pyranonaphthoquinone derivatives alpha-lapachone and beta-lapachone. Cancer Chemother Pharmacol. 2001;47:187–98.
Jackson JK, Higo T, Hunter WL, Burt HM. Topoisomerase inhibitors as anti-arthritic agents. Inflamm Res. 2008;57:126–34.
Tudan C, Jackson J, Higo TT, Burt HM. The effect of inhibiting topoisomerase I and II on the anti-apoptotic response associated with pro-inflammatory crystals of calcium pyrophosphate dihydrate in human neutrophils. Inflamm Res. 2003;52:8–17.
De Miranda FG, Vilar JC, Alves IA, Cavalcanti SC, Antoniolli AR. Antinociceptive and antiedematogenic properties and acute toxicity of Tabebuia avellanedae Lor. ex Griseb. inner bark aqueous extract. BMC Pharmacol. 2001;1:6.
Wanick MC, Bandeira JÁ, Fernandes RV. Ação antiinflamatória e cicatrizante do extrato hidroalcoólico do Líber do Pau d’arco Roxo (Tabebuia avellanedae), em pacientes portadores de Cervicites e Cérvico-vaginites. Rev Inst Antibióticos. 1970;10:41–6.
Liu SH, Tzeng HP, Kuo ML, Lin-Shiau SY. Inhibition of inducible nitric oxide synthase by beta-lapachone in rat alveolar macrophages and aorta. Br J Pharmacol. 1999;126:746–50.
Manna SK, Gad YP, Mukhopadhyay A, Aggarwal BB. Suppression of tumor necrosis factor activated nuclear transcription factor-kB, activator protein-1, c-Jun N-terminal kinase, and apoptosis by β-lapachone. Biochem Pharmacol. 1999;57:763–74.
Smith HS, Smith AR, Seidner P. Painful rheumatoid arthritis. Pain Physician. 2011;14:E427–58.
Bansback NJ, Regier DA, Ara R, Brennan A, Shojania K, Esdaile JM, Anis AH, Marra CA. An overview of economic evaluations for drugs used in rheumatoid arthritis: focus on tumour necrosis factor-alpha antagonists. Drugs. 2005;65:473–96.
Lin HS, Hu CY, Chan HY, Liew YY, Huang HP, Lepescheux L, Bastianelli E, Baron R, Rawadi G, Clément-Lacroix P. Anti-rheumatic activities of histone deacetylase (HDAC) inhibitors in vivo in collagen-induced arthritis in rodents. Br J Pharmacol. 2007;150:862–72.
Klinkhoff A. Biological agents for rheumatoid arthritis: targeting both physical function and structural damage. Drugs. 2004;64:1267–83.
Cavalcante FA, Silva JLV, Carvalho VMN, Camara CA, Silva TM, Pinto AC, et al. Spasmolytic activity of lapachol and its derivatives, alpha and beta-lapachone, on the guinea-pig ileum involves blockade of voltage-gated calcium channels. Rev Bras Farmacog. 2008;18:183–9.
Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–7.
Ough M, Lewis A, Bey EA, Gao J, Ritchie JM, Bornmann W, Boothman DA, Oberley LW, Cullen JJ. Efficacy of b-lapachone in pancreatic cancer treatment. Cancer Biol Ther. 2005;4:95–102.
Guerra AS, Malta DJ, Laranjeira LP, Maia MB, Colaço NC, De Lima MC, Galdino SL, Pitta IR, Gonçalves-Silva T. Anti-inflammatory and antinociceptive activities of indole-imidazolidine derivatives. Int Immunopharmacol. 2011;11:1816–22.
Newbould BB. Chemotherapy of arthritis induced in rats by mycobacterial adjuvant. Br J Pharmacol. 1963;21:127–36.
McCartney-francis N, Allen JB, Mizel DE, Albina JE, Xie QW, Nathan CF, Wahl SM. Suppression of arthritis by an inhibitor of nitric oxide synthase. J Exp Med. 1993;178(2):749–54.
Salvemini D, Wang ZQ, Wyatt PS, Bourdon DM, Marino MH, Manning PT, Currie MG. Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br J Pharmacol. 1996;118:829–38.
Pèrez-gurrero C, Herrera MD, Ortiz R. De sotomayor MA, Fernández MA. A pharmacological study of Cecropia obtusifolia Betrol aqueous extract. J Ethnopharmacol. 2001;76:279–84.
Faria L, Antunes E, Bom C, Araújo AL. Pharmacological characterization of the rat paw edema induced by Bothrops lanceolatus (Fer de lance) venom. Toxicon. 2001;39:825–30.
Prasad N, Gupta A. Fungal peritonitis in peritoneal dialysis patients. Perit Dial Int. 2005;25(3):207–22.
Foster SJ, McCormick ME, Howarth A, Aked D. Leukocyte recruitment in the subcutaneous sponge implant model of acute inflammation in the rat is not mediated by leukotriene B1. Biochem Pharmacol. 1986;35:1709–17.
Kim JY, Hwang YP, Kim DH, Han EH, Chung YC, Roh SH, et al. Inhibitory effect of the saponins derived from roots of Platycodon grandiflorum on carrageenan-induced inflammation. Biosci Biotechnol Biochem. 2006;70:858–64.
Loram LC, Fuller A, Fick LG, Cartmell T, Poole S, Mitchell D. Cytokine profiles during carrageenan-induced inflammatory hyperalgesia in rat muscle and hind paw. J Pain. 2007;8:127–36.
Vigil SVG, Liz R, Medeiros YS, Fröde TS. Efficacy of tacrolimus in inhibiting inflammation caused by carrageenan in a murine model of air pouch. Transpl Immunol. 2008;19:25–9.
Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214:149–60.
Junior VLLP, Rebelo MC, Gomes RN, Assis EF, Neto HCCF, Bóia MN. Il-6 and Il-8 in cerebrospinal fluid from patients with aseptic meningitis and bacterial meningitis: their potential role as a marker for differential diagnosis. Braz J Infect Dis. 2011;15(2):156–8.
Kinne RW, Stuhlmuller B, Burmester GR. Cells of the synovium in rheumatoid arthritis macrophages. Arthritis Res Ther. 2007;9:224–40.
Taylor PC, Feldmann M. Anti-TNF biologic agents: still the therapy of choice for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:578–82.
Grabowski PS, Wright PK, Vanthof RJ, Helfrich MH, Ohshima H, Ralston SH. Immunolocalization of inducible nitric oxide synthase in synovium and cartilage in rheumatoid arthritis and osteoarthritis. Br J Rheumatol. 1997;36:651–5.
Fermor B, Christensen SE, Youn I, Cernanec JM, Davies CM, Weinberg JB. Oxygen, nitric oxide and articular cartilage. Eur Cell Mater. 2007;13:56–65.
Acknowledgments
This work was supported by the Foundation for Science and Technology of Pernambuco-FACEPE and the National Council for Scientific and Technological Development-CNPq, Brazil. The authors are grateful to Sebastien Read-Auber for editing the English.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jerauld Skotnicki.
Rights and permissions
About this article
Cite this article
Sitônio, M.M., de Carvalho Júnior, C.H.R., Campos, I.d.A. et al. Anti-inflammatory and anti-arthritic activities of 3,4-dihydro-2,2-dimethyl-2H-naphthol[1,2-b]pyran-5,6-dione (β-lapachone). Inflamm. Res. 62, 107–113 (2013). https://doi.org/10.1007/s00011-012-0557-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-012-0557-0