Abstract
Background
Tabebuia avellanedae is a tree from the Bignoniaceae family. Commonly know as "pau d'arco" in Brazil, its inner bark is used as analgesic, anti-inflammatory, antineoplasic and diuretic at the Brazilian northeast. A validation of the plant usage has not been previously performed.
Results
Antinociceptive and antiedematogenic effects of Tabebuia avellanedae Lor. ex Griseb. inner bark were measured by nociceptive experimental models in mice. A rat paw edema test induced by carrageenan (1%) was also performed in rats to access the plant's antiedematogenic effect. The inner bark aqueous extract, administered via oral in three different concentration, namely 100, 200 and 400 mg/Kg, reduced the nociception produced by acetic acid (0.6% in water, i.p.) by 49.9%, 63.7% and 43.8%, respectively. The aqueous extract (200 and 400 mg/Kg, p.o.) reduced formalin (1%) effects only at the second phase of the experiment by 49.3% and 53.7%, respectively. Naloxone (5 mg/Kg, i.p.) was not able to revert the extract effect, however caffeine (10 mg/Kg, i.p.) reverted its effect by 19.8% at the second phase of the formalin test. The aqueous extract (200 mg/Kg, p.o.) inhibited edema by 12.9% when we used the rat paw edema model. The acute toxicity was low in mice.
Conclusion
The T. avellanedae inner bark aqueous extract presented antinociceptive and antiedematogenic activities at the used models, with a possible antinociceptive effect associated to the adenosine system.
Similar content being viewed by others
Background
Tabebuia avellanedae is a tree from the Bignoniaceae family. Commonly know as "pau d'arco" in Brazil, its inner bark is used as analgesic, anti-inflammatory, antineoplasic [1, 2] and diuretic at the Brazilian northeast. Its anti-inflammatory, antimicrobial, and antineoplasic activities are cited in the literature as promoted by saponines, flavonoides, cumarines, and natural antibiotics, such as the lapachol and its derivatives encountered on its constituents [3–6]. The antifungal activity of aqueous, dichloromethane and methanol extracts from T. avellanedae and other 13 Paraguayan plants were accessed by Portillo et al. [7]. The aqueous and methanol extracts of T. avellanedae showed the highest antifungal activity. Phytochemical studies revealed the presence of naphthoquinones, anthraquinones and quinoid compounds in T. avellanedae, although lapachol, previously cited as anti-inflammatory [8], was not detected in its aqueous extract [9]. Since T. avellanedae inner bark is extensively used as analgesic in Brazil, the goal of our work was to describe the antiedematogenic and antinociceptive effects and acute toxicity of T. avellanedae inner bark aqueous extract on various animal models.
Results
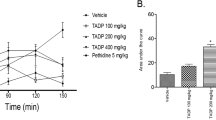
The inhibition of rat paw edema induced by carrageenan was significantly different between all groups (ANOVA: F0.05;3;140 = 45.72; p < 0.0005) and time intervals tested (ANOVA: F0.05;4;140 = 35.66; p < 0.0005) The volume variation was not proportional between all groups (ANOVA: F0.05;12;140 = 1.94; p < 0.05). The T. avellanedae aqueous extract inhibited edema only at the 200 mg/Kg dose (Tukey: q0.05;140;4 = 5.02; p < 0.05, Table 1). This test is used to evaluate anti-inflammatory drugs and has been used to test the antiedematogenic effect of various substances [10].
When performing an writhing test, results showed notable differences at the writhing frequency between all tested groups (Kruskal-Wallis: F0.05;5;47 = 9.26; p < 0.0005). T. avellanedae (100, 200 and 400 mg/Kg, p.o.) reduced the acetic acid effect (Nemenyi: q0.05;∞;6 = 4.28, p < 0.05 and q0.05;∞;6 = 4.17, p < 0.05, respectively, Table 2). The abdominal constriction response is an unspecific nociception model used to evaluate the central and peripheral analgesics drug activities [11].
Our experiments showed significant differences between four groups when the reaction time was taken into account at the formalin 1% test during the first and second phases (Kruskal-Wallis: F0.05;4;34 = 22.52; p < 0.0005 and F0.05;4;34 = 23.45; p < 0.0005, respectivelly). The aqueous extract of T. avellanedae (200 e 400 mg/Kg) reduced the formalin effect only at the second phase (Nemenyi: q0,05;∞;5 = 4.264, p < 0.05 and q0,05;∞;5 = 4.643, p < 0.05, Table 3).
Naloxone (5 mg/Kg, i.p.) did not revert the action of the aqueous extract (400 mg/Kg) (Nemenyi: q0.05;∞;3 = 4.27; p < 0.05). Caffeine, an adenosine A1 and A2 receptors antagonist, reverted the T. avellanedae aqueous extract action at the formalin test second phase (Nemenyi: q0.05;∞;3 = 1.45; p > 0.05, Table 3). There was no LD50 when using doses up to 5000 mg/Kg.
Discussion
The aqueous extract at 200 mg/Kg inhibited the rat paw edema induced by carrageenan in a similar way as indomethacin (Table 1). However at 400 mg/Kg we did not detect edema reduction. It would be expected a dose-dependent effect when using higher concentrations. Since the aqueous extract contains several compounds, another compound in the extract may be acting simultaneously to revert its own action.
Although the abdominal writhes induced by acetic acid represent a peripheral nociception model [12], this is not a specific model, since several compounds, such as, tricyclic antidepressants [13] and anti-histaminic [14] inhibit the writhes induce by acetic acid. Even though the T. avellanedae AE prevented the writhes, there is still the need for studies using other analgesia models.
Three distinct phases are involved on the acute vascular response. At the first phase simultaneous release of histamine and serotonin occurs. At the second phase kinin release, such as bradykinin, occurs and at the terminal phase prostaglandins release occurs [15]. Any substance inhibiting the carrageenan action at the used model is considered as having anti-inflammatory action.
Since the effect of the AE was not reverted by naloxone at the formalin test second phase, our studies indicate that the opioid system does not participate at the AE (200 and 400 mg/Kg) pharmacological properties. On the other hand caffeine reverted the AE action at the formalin test second phase, suggesting the participation of the adenosine system at the analgesic effect. Adenosine may interact with at least four subtipes of receptors coupled with protein G, namely A1, A2a, A2b, and A3[16]. There has been evidences that activation of the A1 and A2 receptors produce an antinociceptive effect, while activation of the A3 receptor produce nociception [17]. These facts are supported by experiments where caffeine produced antinociception [16]. Henceforth the AE antinociceptive effect at the formalin test second phase may be associated to an activation of the A1 and/or A2 adenosine receptors. Further studies are needed to confirm these findings.
The formalin test is one of the most used models to explain pain and analgesia mechanisms, with better results than the ones using mechanical or thermal stimulus [18]. This model is constituted by two distinct phases. The first phase represents the irritating effect of formalin at the sensorial fibers-C. The second is an inflammatory pain response. Central acting analgesics, such as morphine, inhibit both phases. Peripheral acting drugs, such as non-steroid anti-inflammatory and corticosteroids, inhibit only the second phase [19].
It was not possible to measure the plant LD50 due to the lack of mice deaths even at high concentration of the T. avellanedae aqueous extract. This result indicates that the plant has a low toxicity profile [20]. The plant presented antiedematogenic and antinociceptive at the peripheral system, probably due to its action at mediators, such as bradykinin and prostaglandins at the neuron termination. Hiperalgesy may also be involved on this process, mediated by the adenosine A2 receptor [21, 22].
Conclusion
Tabebuia avellanedae is used by popular medicine as anti-inflammatory, antimicrobial, antineoplasic, and diuretic. The T. avellanedae inner bark aqueous extract presented antinociceptive and antiedematogenic activities at the used models, with the antinociceptive effect associated to the adenosine system. These results validate the plant popular use as analgesic and antiinflammatory. The antimicrobial, antineoplasic, and diuretic activities were not studied.
Materials and Methods
Plant material
The Tabebuia avellanedae inner bark collected at the "Capim Grosso" village (Canindé do São Francisco region) was used. The plant identification was made on site by our herbalist (Gilvane V. Souza, Biology Department) and a voucher specimen number 2955 was deposited at the University of Sergipe herbarium, (Universidade Federal de Sergipe, CCBS, Departamento de Biologia, São Cristovão, Sergipe, 49100–000, Brazil).
Aqueous extract preparation
The plant inner barks were dried at 40°C in oven and triturated using a mill in order to obtain a powder. Distilled water (1:10 w/v) at 100°C was added to the triturated powder, constituting the aqueous extract. The extract was infused for 30 min, filtered, lyophilized and used on the pharmacological tests. The yield of the aqueous extract was 8.9% w/w. In order to perform all experiments the extract was reconstituted in enough water to make a 100 mg/mL solution and administered p.o. 60 minutes before the experiment.
Animals
Swiss mice (20–35 g) and Wistar rats (120–200 g) male and female were used as test animals. The animals were maintained in plastic boxes, with food and water ad libitum. The animals submitted to oral administration of the extract or drugs were fasted for 12 hours.
Drugs preparation
Drugs used in the experiments were diluted in a way to obtain a injection volume of 0.1 mL/10 g (animal weight), except when defined in the text. Each drug was dissolved in appropriate solvents as follows: Indomethacin (Sigma), diluted in water/0.1 N NaOH (pH = 8); carrageenan 1% (Sigma), in saline solution; acetic acid 0.6% (Merck), in water; Morphine hydrochloride (Sigma) in water; formalin 1% (Baker) in water; naloxone hydrochloride (Sigma), in water; caffeine (Sigma) in water.
Acute toxicity (LD50)
In order to verify the plant LD50, mice (n = 10) received the aqueous extract (1, 3 and 5 g/Kg; p.o.). The mortality index was observed during 48 hours [20].
Rat paw edema test
The antiedematogenic activity was evaluated by the rat paw edema test[10] (n = 8) induced by carrageenan at 1% in. Indomethacin (10 mg/Kg; p.o.) and the T. avellanedae aqueous extract (200 and 400 mg/Kg; p.o.) were administered 1 h before the flogistic agent subplantar injection. The control group received only the carrageenan injection. The paw volume was measured at the time 0, then measured again 1, 2, 3, and 4 h after the carrageenan administration by the water displacement method measured with the help of a plethysmometer (model 7150, Ugo Basile).
Writhing test
The antinociceptive effect was evaluated by the writhing test [11] in mice (n = 9), induced by acetic acid 0.6% (0.1 mL/10 g; i.p.). The T. avellanedae aqueous extract (100, 200 and 400 mg/Kg; p.o.) was administered 1 h before the nociceptive agent. Ten minutes after the acid administration we observed the number of writhes for a period of 20 min. Morphine (2.5 mg/Kg, i.p.) and indomethacin (10 mg/Kg, p.o.) were used as test standards.
Formalin test
The antinociceptive effect was also evaluated by the formalin test. It was observed the time (seconds), which each mice (n = 8) spent licking its posterior left paw after administration, subplantar route, of formalin 0.02 mL, 1% [19, 23]. The T. avellanedae aqueous extract (100, 200 and 400 mg/Kg; p.o.) was administered 1 h before the formalin injection. The standard test drug was morphine (7.5 mg/Kg, i.p.). The reaction time to pain was measured during 0–5 min and 20–25 min after the stimulus. In order to verify a possible opioid system participation, naloxone (5 mg/Kg; i.p.) was administered with the AE to another animal group (n = 8, Table 3). In a similar way caffeine (10 mg/Kg; i.p.) was administered with the AE to verify possible adenosine system receptor participation. Morphine and naloxone were also administered to another animal group.
Statistical analysis
The inflammation test results were analyzed by ANOVA followed by the Tukey test. In order to analyze the nociception model we used the non-parametrical analogous test, the Kruskal-Wallis followed by the Nemenyi test, with a significance level of 5%. Inhibition percents were calculated by the formula: Inhibition percent = (1 -Vt/Vc)×100, where Vt and Vc represent the average paw volume, the number of writhes, or the licking paw time of the treated and control groups, respectively.
References
Ueda S, Umemura T, Dohguchi K, Matsuzaki T, Tokuda H, Nishino H, Iwashima A: Production of anti-tumour-promoting furanonaphthoquinones in Tabebuia avellanedae cell cultures. Phytochemistry. 1994, 36: 323-325. 10.1016/S0031-9422(00)97069-9.
de Santana CF, de Lima O, d'Albuquerque IL, Lacerda AL, Martins DG: Antitumoral and toxicological properties of extracts of bark and various wood components of Pau d'arco (Tabebuia avellanedae). Rev. Inst. Antibiot. (Recife). 1968, 8: 89-94.
Almeida ER: Plantas medicinais brasileiras: Conhecimentos populares e científicos: HEMUS. 1834
Falkenberg MB: Quinonas. In: Farmacognosia - da planta ao medicamento Edited by UFRGS/UFSC. pp. 821. Porto Alegre: Editora da UFRGS;. 1999, 821-
Panizza S: Plantas que curam: cheiro de mato. São Paulo: IBRASA. 1997
Steinert J, Khalaf H, Rimpler M: HPLC separation and determination of naphto-[2,3,6]-furan-4,9-diones and related compounds in extracts of Tabebuia avellanedae (Bignoniaceae). J. Chromatogr. A. 1995, 693: 281-287. 10.1016/0021-9673(94)01128-2.
Portillo A, Vila R, Freixa B, Adzet T, Canigueral S: Antifungal activity of Paraguayan plants used in traditional medicine. J. Ethnopharmacol. 2001, 76: 93-98. 10.1016/S0378-8741(01)00214-8.
de Almeida ER, da Silva Filho AA, dos Santos ER, Lopes CA: Antiinflammatory action of lapachol. J. Ethnopharmacol. 1990, 29: 239-241. 10.1016/0378-8741(90)90061-W.
Steinert J, Khalaf H, Rimpler M: High-performance liquid chromatographic separation of some naturally occurring naphthoquinones and anthraquinones. J. Chromatogr. A. 1996, 723: 206-209. 10.1016/0021-9673(95)00841-1.
Winter CA, Risley EA, Nuss GW: Carrageenan-induced edema in hind paw of the rat as a assay for antiinflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 111: 547-547.
Collier HO, Dinneen LC, Johnson CA, Schneider C: The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br. J. Pharmacol. 1968, 32: 295-310.
Wei ET, Kiang JG, Buchan P, Smith TW: Corticotropin-releasing factor inhibits neurogenic plasma extravasation in the rat paw. J. Pharmacol. Exp. Ther. 1986, 238: 783-787.
Takahashi RN, Paz MM: Influence of naloxone on analgesic effects of antidepressants in mice. Braz. J. Med. Biol. Res. 1987, 20: 607-610.
Yeh SY: Potentiation of pentazocine antinociception by tripelennamine in the rat. J. Pharmacol. Exp. Ther. 1985, 235: 683-689.
Di Rosa M, Giround JP, Willoughby PA: Studies on the mediators of acute inflammatory response induced in rat in different sites by carrageenan and tupentine. J. Pathol. Bacteriol. 1971, 104: 5-29.
Fredholm BB, Gustafsson LE, Hedquist P, Sollevi A: Adenosine in the regulation of neurotransmitter release in the peripheral nervous system. In: Regulation functions of adenosine Edited by Berne RM, Rall TW, Rubio R. pp. 479–493. Boston: Martinus Nijhoff;. 1983, 479-493.
Sawynok J: Purine and nociception. In: Purinergics approaches in experimental therapeutics Edited by Jacobson K, Jarvis MF. pp. 495–513. New York: Wiley & Sons;. 1997, 495-513.
Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K: The formalin test: an evaluation of the method. Pain. 1992, 51: 5-17. 10.1016/0304-3959(92)90003-T.
Husnkaar S, Hole K: The formalin test in mice: dissociation between inflammatory and non-inflamamatory pain. Pain. 1987, 30: 103-119.
Lorke D: A new approach to practical acute toxicity testing. Arch. Toxicol. 1993, 54: 275-287.
Sawynok J, Yaksh TL: Caffeine as an analgesic adjuvant: a review of pharmacology and mechanisms of action. Pharmacol. Rev. 1993, 45: 43-85.
Daval JL, Nehlig A, Nicolas F: Physiological and pharmacological properties of adenosine: therapeutic implications. Life Sci. 1991, 49: 1435-1453. 10.1016/0024-3205(91)90043-B.
Dubuisson D, Dennis SG: The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977, 4: 161-174. 10.1016/0304-3959(77)90130-0.
Acknowledgements
We would like to acknowledge CNPq, CNPq-PIBIC, and BNB for supporting grants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
de Miranda, F.G.G., Vilar, J.C., Alves, I.A.N. et al. Antinociceptive and antiedematogenic properties and acute toxicity of Tabebuia avellanedae Lor. ex Griseb. inner bark aqueous extract. BMC Pharmacol 1, 6 (2001). https://doi.org/10.1186/1471-2210-1-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2210-1-6